Lacking Overall IMspiration for Triplet Therapy in Melanoma? A Review of the Interim Analysis of IMspire150.
Featured Article
Overall survival with first-line atezolizumab in combination with vemurafenib and cobimetinib in BRAFV600 mutation- positive advanced melanoma (IMspire150): second interim analysis of a multicentre, randomised, phase 3 study. Ascierto et al. Lancet Oncology 2023 January; 24(1):33-44 8;387(23):2113-2125.
Introduction
On February 3rd, 2023 the multi-institutional Community of Cutaneous Oncology (CCO) Journal Club reviewed the recently published Lancet Oncology article “Overall survival with first-line atezolizumab in combination with vemurafenib and cobimetinib in BRAFV600 mutation- positive advanced melanoma (IMspire150): second interim analysis of a multicentre, randomised, phase 3 study”(Ascierto et al. 2023). Participants included clinicians and investigators from Massachusetts General Hospital, Mass Eye and Ear, Brigham and Women’s Hospital, the National Institutes of Health, George Washington Medical Center, and Pennsylvania Hospital. Importantly, the comments in this article represent the views of the authors of this Perspectives on the Science piece after the Journal Club. It does not represent views of any other members of the CCO or the affiliated institutions. In this article we provide a summary of the discussion regarding this important contribution to the literature.
Background for the Study
The treatment landscape for advanced melanoma has changed rapidly over the last ten years (Figure 1)(D. M. Miller et al. 2022). Options for patients with advanced disease include immune-directed therapies, such as anti-PD-1(L1), anti-CTLA-4 and anti-LAG-3 monoclonal antibodies, as well as oncogene-pathway targeted therapies (TT) for patients with actionable mutations, including kinase inhibitors directed at BRAF and MEK (D. Miller, Flaherty, and Tsao 2014). Although dual BRAF/MEK inhibition (BRAFi/MEKi) is generally associated with higher response rates (Figure 2), immune checkpoint inhibitors (ICI) have become a preferred first-line strategy for most patients with advanced disease due to the potential for durable responses (Figures 2, 3 and 4).
Unfortunately, therapeutic options in the second-line setting have yielded sub-optimal results (Kim et al. 2023), highlighting the need to optimize front-line therapy. Given the potential to improve the response rates associated with ICIs and the durability of oncogene-pathway directed therapy, efforts to combine immunotherapy with targeted therapy have been ongoing for over a decade. Mechanistic rationale for combining BRAFi/MEKi with immune checkpoint inhibitors includes priming of the tumor microenvironment for immunotherapy via upregrulation of programmed death 1 ligand (PD-L1), increasing lymphocyte traffic and reduction of anti-inflammatory cytokines(Frederick et al. 2013; Wilmott et al. 2012; Schilling and Paschen 2013; Long et al. 2013; Boni et al. 2010; Kono et al. 2006; Sapkota, Hill, and Pollack 2013; Vella et al. 2014; Cooper et al. 2013). Initial investigations combining ICI with TT, however, highlighted the potential risk of enhanced toxicity. One early study of combination ipilimumab and vemurafenib reported the development of hepatoxicity in a significant portion of patients(Ribas et al. 2013). Subsequent studies demonstrated that BRAFi/MEKi could be safely administered with anti-PD-1(L1) monoclonal antibodies (Sullivan et al. 2019; Ferrucci et al. 2020; Dummer et al. 2022). IMspire150, a double-blinded, randomized, placebo-controlled, demonstrated improved median progression-free survival (PFS) of combination vemurafenib/cobimetinib/atezolizumab (vem/cobi/atezo) compared with vemurafenib/cobimetinib (vem/cobi) alone (15.1 vs 10.6 months HR: 0.78 (95% CI 0.63 - 0.97))(Gutzmer et al. 2020). Triplet therapy (BRAFi/MEKi/αPD-L1) was associated with more grade 3 or greater treatment-related adverse events (TRAEs) (Figure 5). However, the risk-to-benefit trade off was deemed acceptable and these results led to the FDA approval of this triplet therapy on July 30, 2020.
Additional investigations have evaluated the safety and efficacy of triplet BRAFi/MEKi/αPD-1(L1) therapy. KEYONTE-022, a phase 2 trial of dabrafenib/trametinib (dab/tram) plus pembrolizumab vs. dab/tram/placebo, demonstrated improved PFS, OS and duration of response (DOR) with triplet therapy (Ferrucci et al. 2020). Notably DOR was more than double with triplet therapy (25.1 vs. 12.1 months; HR = 0.32) compared with doublet TT. Similarly in COMBI-i, a phase III trial evaluating the anti-PD-1 mAb spartalizumab plus dab/tam, demonstrated improved PFS with triplet therapy compared to BRAFi/MEKi (16.2 vs. 12.0 months; HR=0.82 (95%CI 0.66 to 1.03)), although that endpoint was not statistically significant(Dummer et al. 2022). Taken collectively, triplet therapy with BRAFi/MEKi/αPD-1(L1) likely improves PFS compared to BRAFi/MEKi (Figure 6), but at the expense of enhanced toxicity (Figure 7). The effect on overall survival (0S) has less supporting data. The interim results of IMspire150 report on that important endpoint.
Study Design
IMspire150 is a multi-center, phase III randomized study. 514 patients with advanced BRAF V600-mutant melanoma were randomized into treatment groups consisting of cobimetinib 60 mg once daily with vemurafenib 960 mg twice daily for 21 days followed by vemurafenib 720 mg twice daily(triplet therapy group) or 960 mg twice daily (doublet therapy group) for 7 days (Figure 8). The triplet therapy group then received atezolizumab from cycle 2 and beyond. The doublet therapy group instead received placebo from cycle 2 onwards. As previously published, the primary endpoint was investigator-assessed PFS, with OS as a prespecified interim analysis. OS is the focus of this most recent report. Hazard Ratios (HRs) for PFS and OS survival were estimated using a stratified Cox model.
Figure 8. IMspire150 Study Design
Study Results
At the data cutoff of September 8th, 2021, 273 patients who entered the study ha died. 126 deaths occurred in the triplet therapy group compared with 147 in the control group. Median overall survival was 39.0 months in the treatment group compared with 22.8 months in the control group. The Cox model HR for OS was 0.84 (95% CI 0.66 - 1.06, p = 0.14). Median PFS was 15.1 vs. 10.6, in favor of vem/cobi/atezo group. The most common cause of death was disease progression. No new safety signals were reported.
Discussion
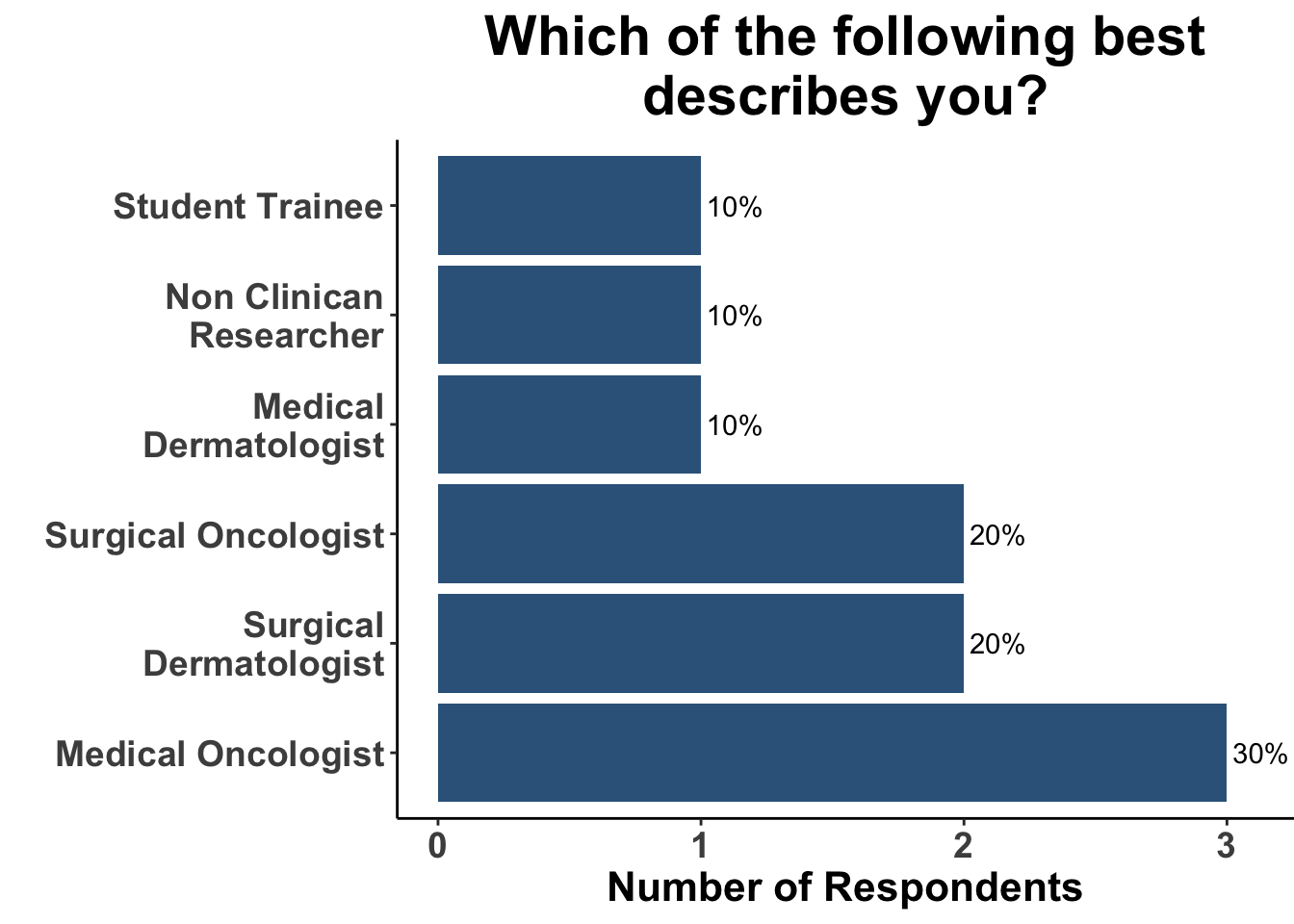
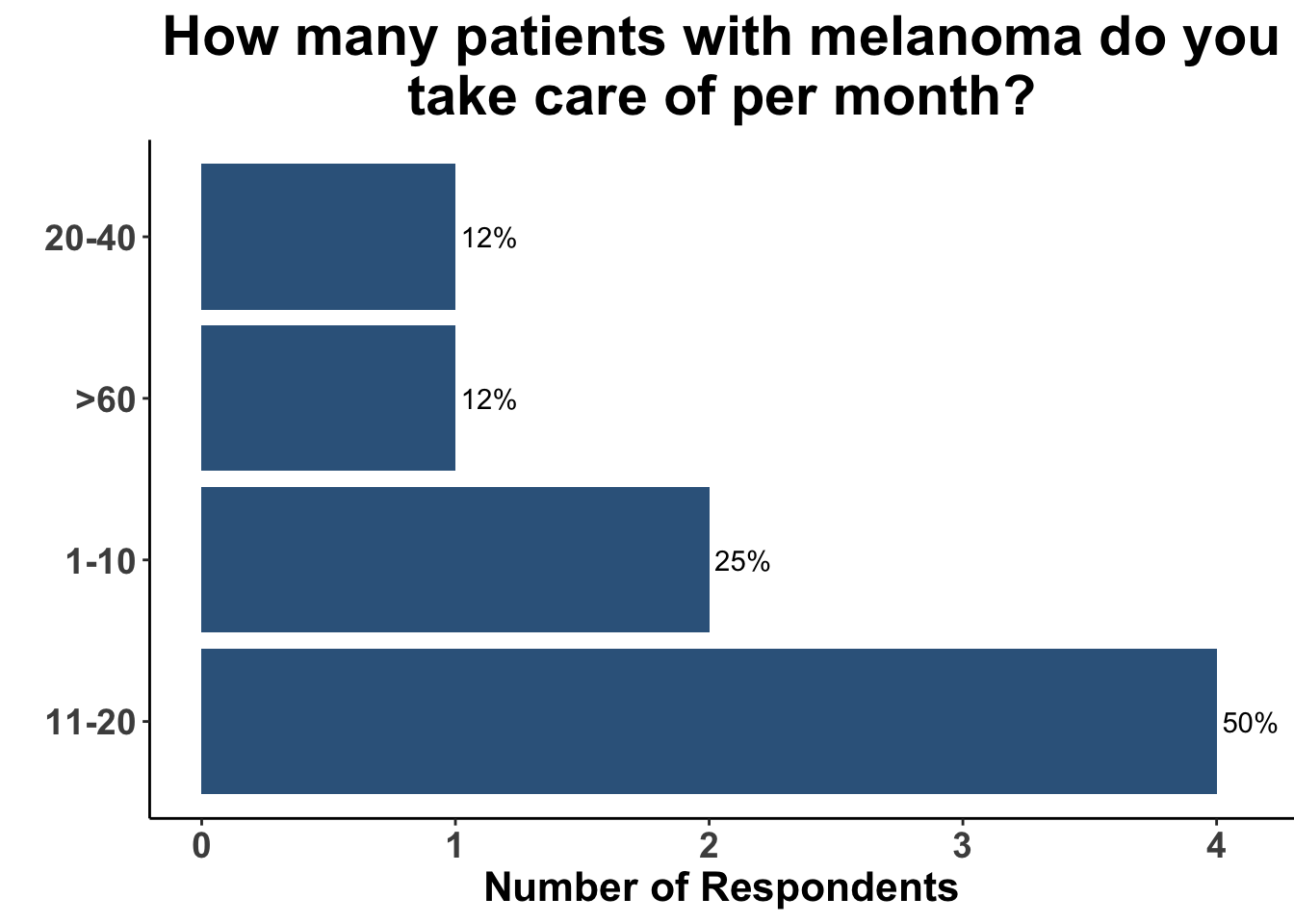
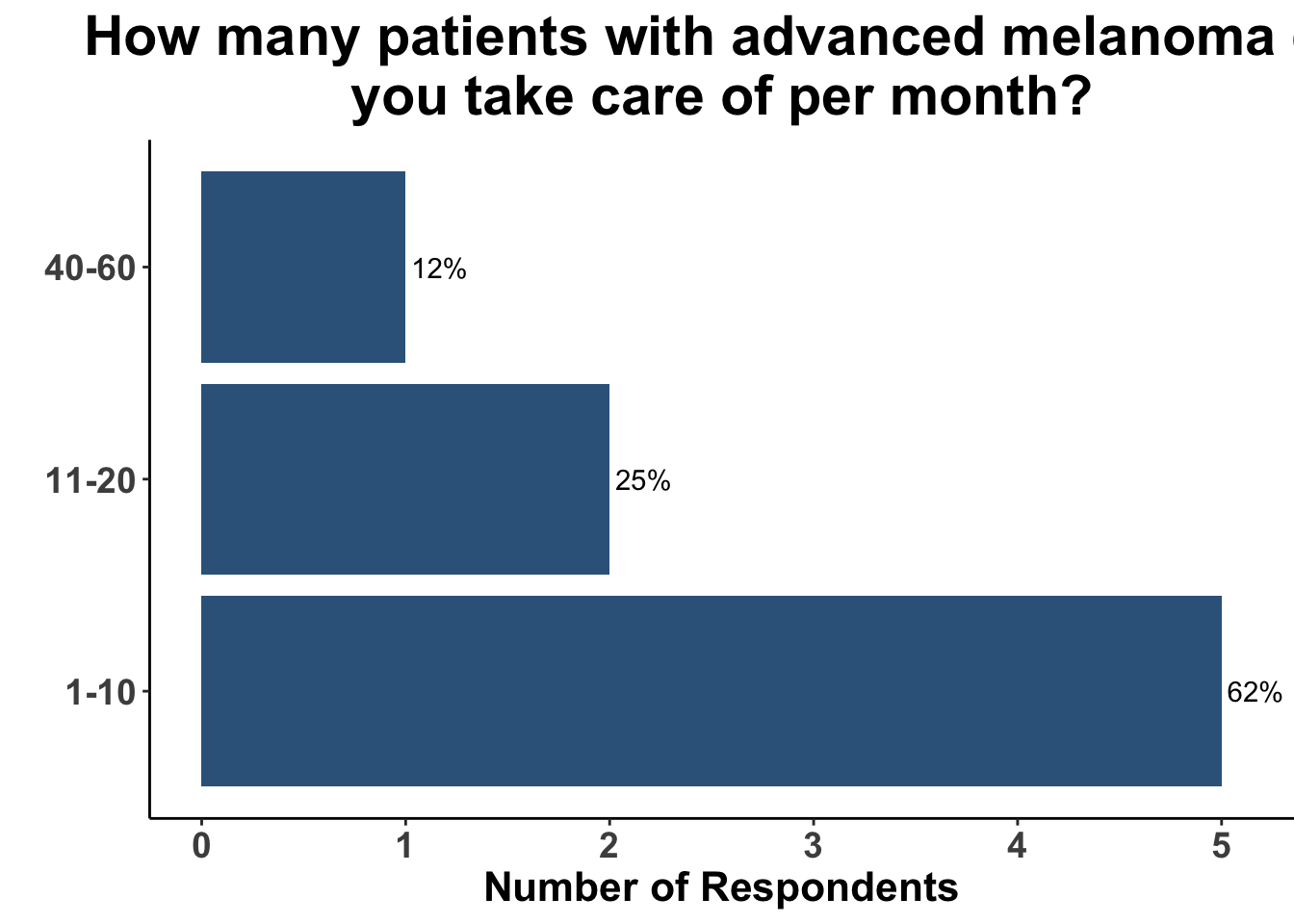
Due to the rapid evolution in treatment for melanoma, clinicians have a variety of front-line options for advanced disease. A survey of the multi-institutional and multi-disciplinary group of CCO members present at the February 3rd Journal Club (Figures 9-11) underscores the diversity of current approaches used by melanoma oncologists.
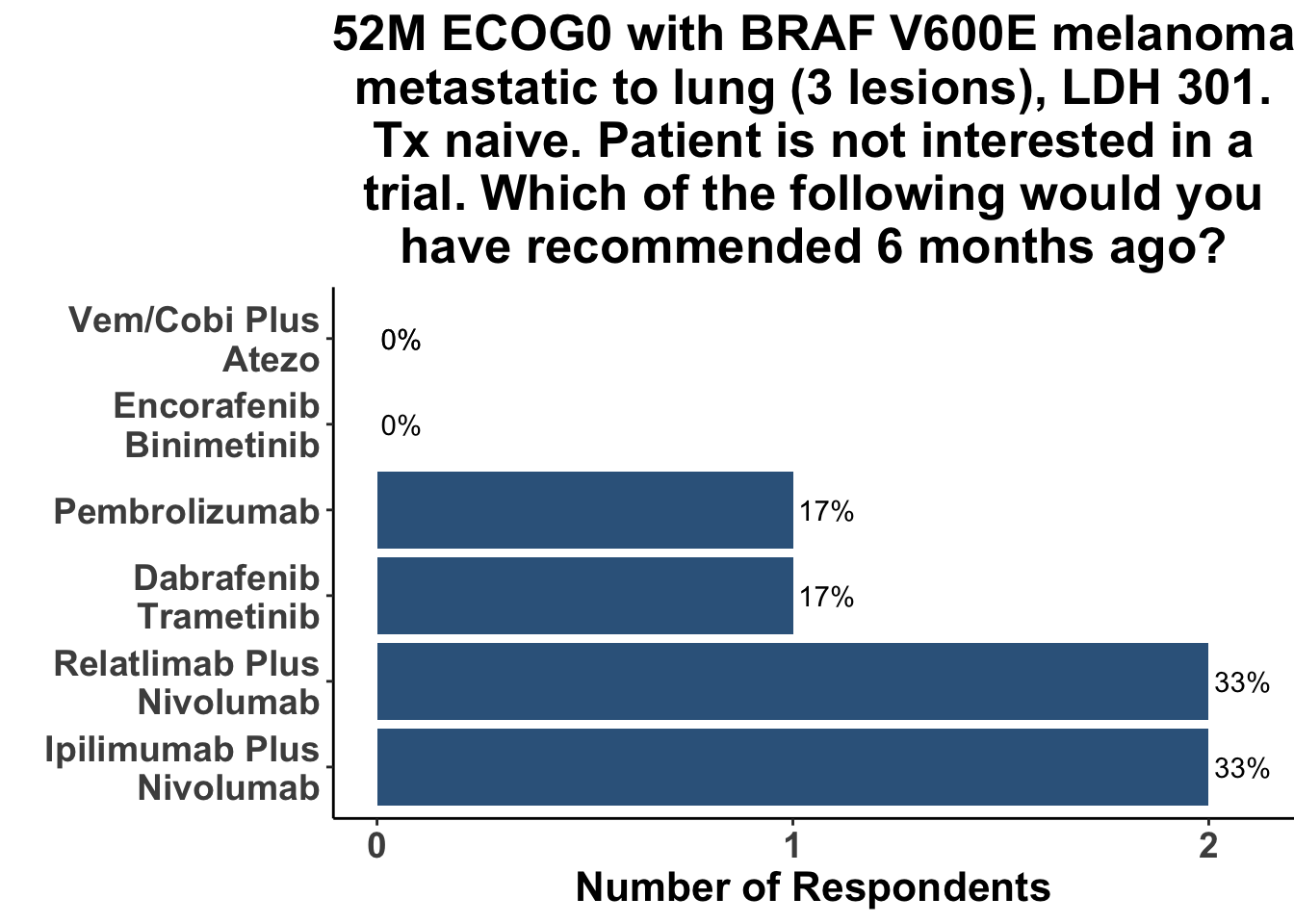
When presented with a case of oligometastatic BRAF V600E melanoma, dual immune checkpoint inhibition was the most commonly recommended approach, with a third of respondents stating they would choose an αCTLA4/αPD-1 regimen, and another third recommending an αLAG-3/αPD-1 regimen. Only one respondent favored single agent αPD-1(L1), with one other selecting a BRAFi/MEKi-based strategy (Figure 12).
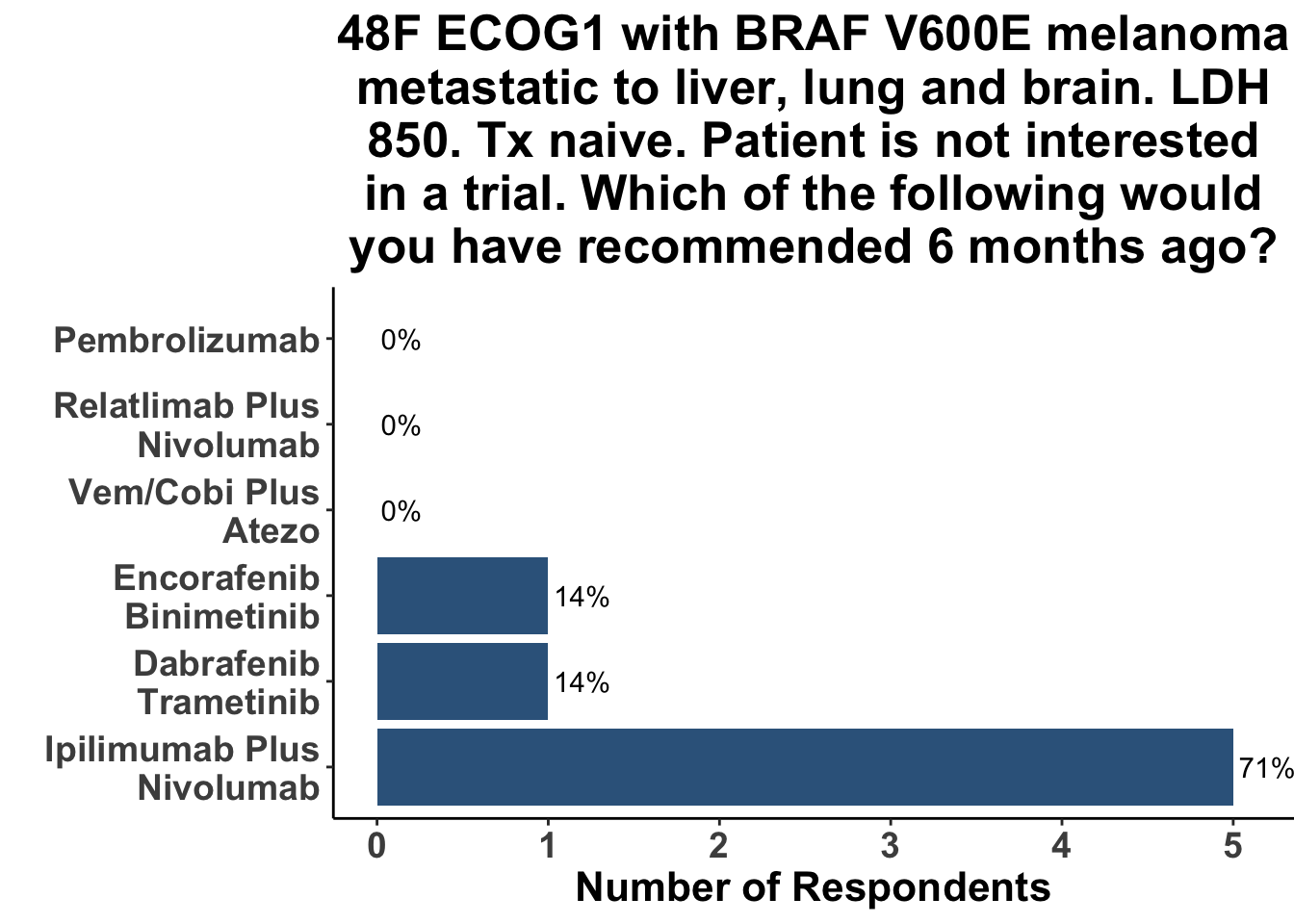
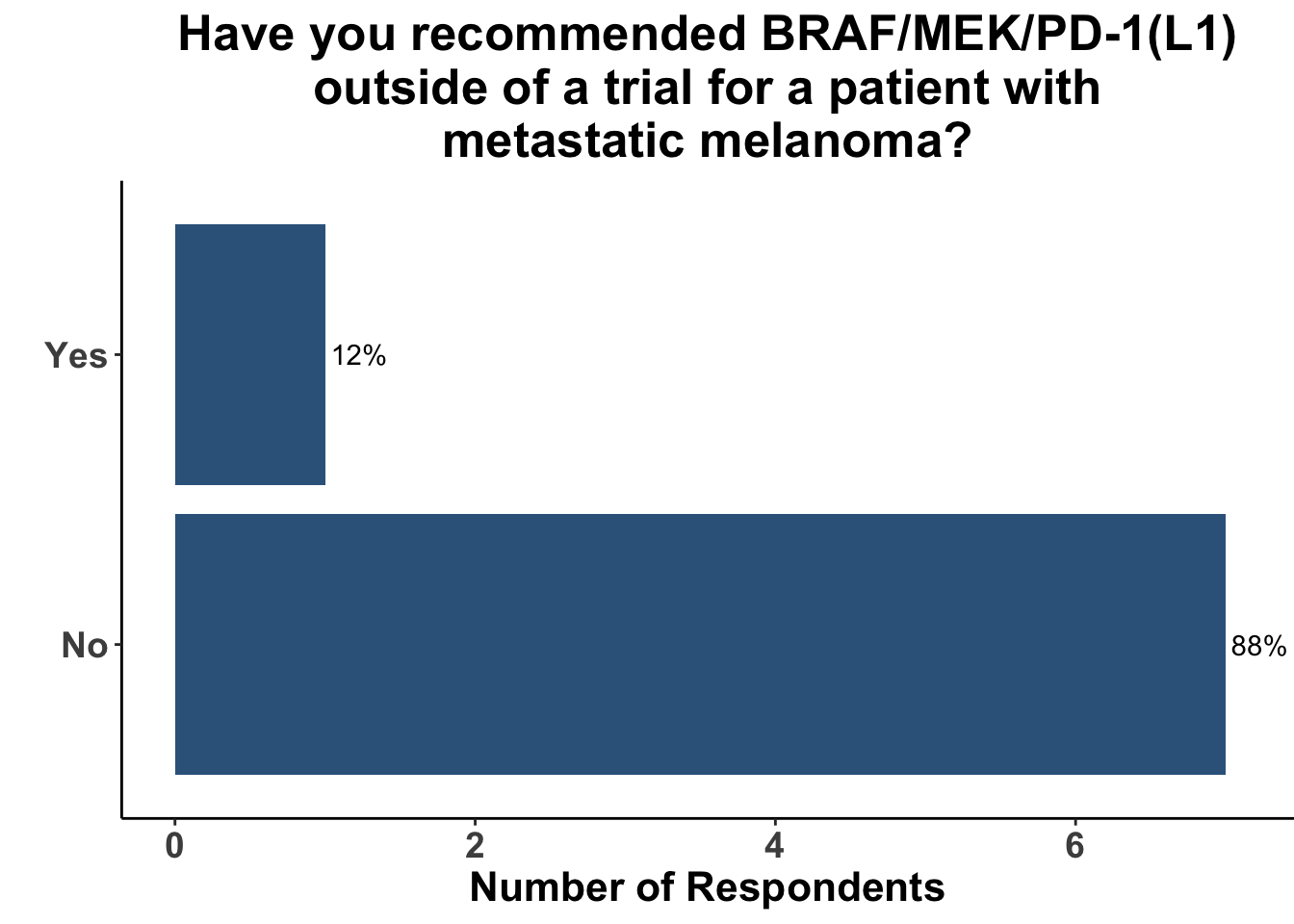
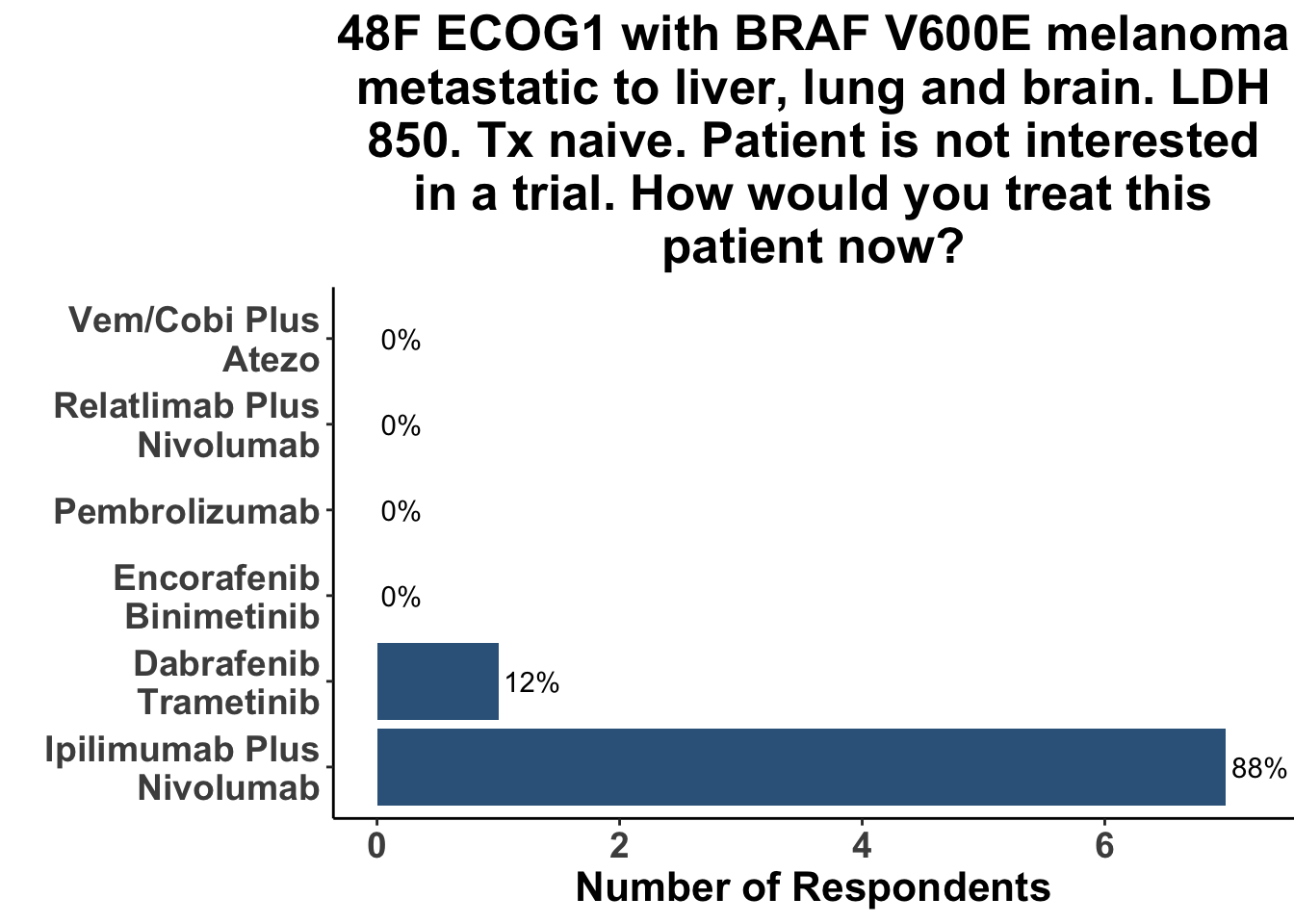
Not surprisingly, the majority of melanoma clinicians (71%, 5/7) selected an αCTLA4/αPD-1 based strategy for a patient with more significant tumor burden, including intracranial disease (Figure 13). CCO members cited the results from CheckMate-204 as rationale for front-line Ipi/Nivo for patients with brain metastases (Hussein A. Tawbi et al. (2018)). None of the attendees would have selected a regimen including both BRAFi/MEKi and immunotherapy six months ago (i.e. before the results of this paper) for either clinical scenario. Indeed, the overall experience with triplet therapy was limited, with only one practioner having recommended BRAFi/MEKi/αPD-1 outside of a clinical trial previously (Figure 14).
For many years there was little high-quality data guiding clinician’s initial treatment strategy selection for patients with advanced BRAF V600 mutant disease. Due to the potential of durable responses with immunotherapy, ICI was often chosen as the initial approach despite not having randomized, controlled data to support this decision. However, data from the recently published Phase III DREAMseq trial has helped fill this important information gap. Two-year OS rates were superior for BRAF V600 mutant patients treated initially with αCTLA4/αPD-1 compared with front-line BRAFi/MEKi (71.8% vs 51.5%, p = 0.01) (Atkins et al. 2023). Median duration of response (mDOR) was also significantly greater with upfront ICI compared with initial TT (mDOR not reached for the initial Ipi/Nivo group vs. 12.7 months for the initial BRAFi/MEKi group, p <0.001). These data reinforce the importance of sequencing therapy appropriately in patients with advanced disease.
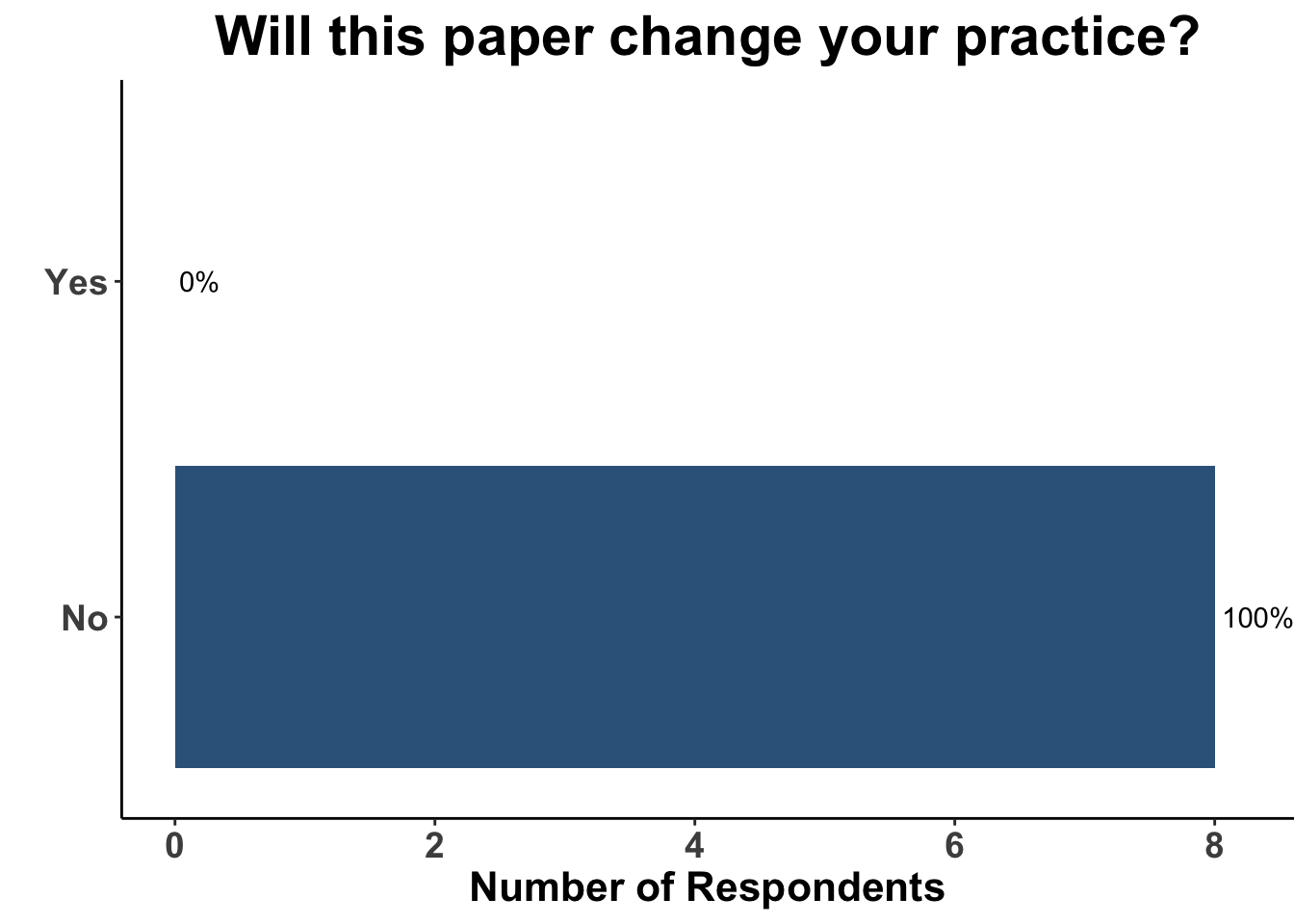
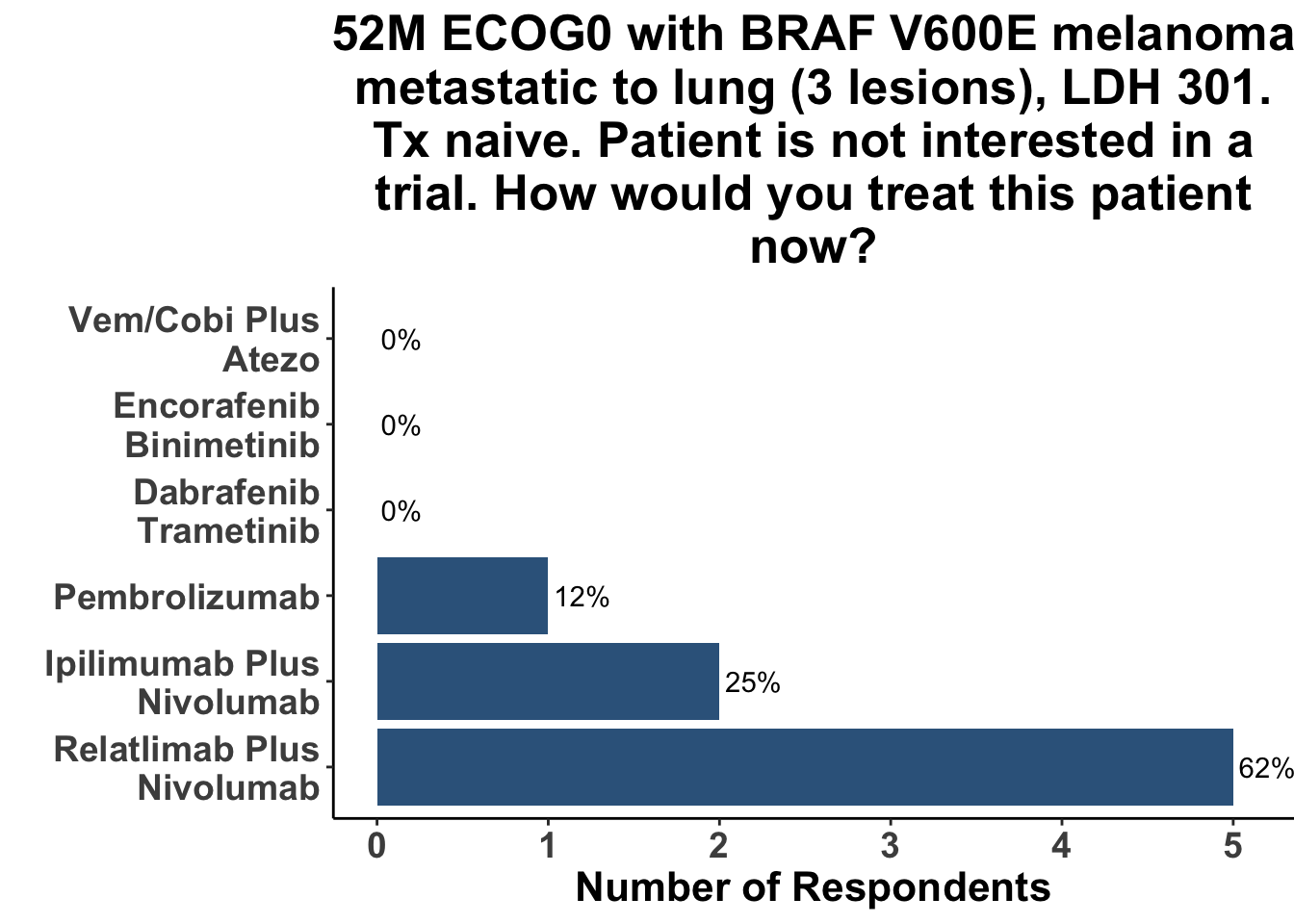
The totality of the evidence from IMspire150, Keynote-022 and COMBI-i suggests that the addition of αPD-1(L1) therapy to a BRAFi/MEKi backbone yields improvement in PFS (and possibly OS) at a cost of added toxicity. The major limitation of these studies is that in the current treatment environment - largely solidified by the DREAMseq trial - the control groups (BRAFi/MEKi) are no longer a preferred initial regimen for the majority of patients with advanced, untreated BRAF V600 melanoma. Thus, despite a signal in all three studies that adding αPD-1(L1) to BRAFi/MEKi improves patient outcomes, the role of triplet therapy in the first-line setting remains uncertain. A more relevant and clarifying comparator of front-line triplet therapy would be either monotherapy αPD-1(L1) or dual ICB, given that ICI is being used in the initial management plan for most patients. The operative question being whether adding targeted therapy to an immunotherapy-based regimen produces improved outcomes compared to a front-line immunotherapy-only strategy. As a consequence of this important caveat, the borderline insignificant interim results of IMspire150 had little effect on the medical decision making of the CCO JC attendees (Figure 15). Given that the study was not answering a question critically important to practioners in 2023, the impact of the paper would have likely been the same had the hazard ratio for OS excluded the null hypothesis. To further support the lack of clarity on the role of triplet therapy after these interim results, respondents replied with similar treatment approaches to patients with oligo- (Figure 16) and more widespread (Figure 17) disease in the current day, as compared with six months ago (Figures 12-13). Again, none of the practioners selected triplet therapy for these clinical scenarios after discussing the recent update of IMspire150.
Although the clinical vignette of high-tumor burden disease did not elicit a selection of triplet therapy by any of the melanoma clinicians, the question of whether triplet therapy or even quadruple therapy (i.e. BRAFi/MEKi/αPD-1/αCTLA4) would be used in the case of impending visceral crisis was raised. Indeed, the one clinician with previous real-world experience with triplet therapy utilized concomitant BRAFi/MEKi/αPD-1 therapy in a patient with fulminant disease including widespread liver metastases and diffuse melanosis cutis. Fortunately that patient went on to have a complete response and is alive today. Patients that present with symptomatic brain metastases represent another cohort that may benefit from combination TT/ICI. While combination CTLA4/αPD-1 has shown impressive activity in patients with asymptomatic intracranial metastases, the efficacy of ipi/nivo is significantly attenuated in patients receiving corticosteroids for symptomatic disease(Hussein A. Tawbi et al. 2021). Therefore, there is rationale to treat with a lead-in of targeted therapy to decrease intracranial disease burden in order to expedite steroid tapering prior to the administration of immunotherapy. Thus, the discussion reinforced the importance of having options in the treatment armamentarium which can be applied on a case-by-case basis.
Materials and Methods
This Perspectives on the Science piece was published using Quarto®. The survey was conducted using REDCap® (Harris et al. 2009). The figures depicting the survey data were created using R (version 4.0.0) and the tidyverse suite of packages (Wickham et al. 2019), including ggplot2 (Wickham 2016). The figure depicting FDA approved therapies in melanoma was created using the skincancerRx package (D. Miller and Shalhout 2022).
Bibliography
Appendix
Citation
@article{m.miller2023,
author = {David M. Miller and Kevin S. Emerick and Vishal A. Patel and
Sonia Cohen and Isaac Brownell and Donald P. Lawrence},
publisher = {Community of Cutaneous Oncology},
title = {Lacking {Overall} {IMspiration} for {Triplet} {Therapy} in
{Melanoma?} {A} {Review} of the {Interim} {Analysis} of
{IMspire150.}},
journal = {Journal of Cutaneous Oncology},
volume = {1},
number = {1},
date = {2023-02-04},
url = {https://journalofcutaneousoncology.io/perspectives/triplet_therapy_for_advanced_melanoma/},
issn = {pending},
langid = {en}
}