| USPSTF Grade Definitions | ||
| Grade | Definitions | Suggestions for Practice |
|---|---|---|
| A | The USPSTF recommends the service. There is high certainty that the net benefit is substantial. | Offer or provide this service. |
| B | The USPSTF recommends the service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. | Offer or provide this service. |
| C | The USPSTF recommends selectively offering or providing this service to individual patients based on professional judgment and patient preferences. There is at least moderate certainty that the net benefit is small. | Offer or provide this service for selected patients depending on individual circumstances. |
| D | The USPSTF recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. | Discourage the use of this service. |
| I | The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. | Read the clinical considerations section of USPSTF Recommendation Statement. If the service is offered, patients should understand the uncertainty about the balance of benefits and harms. |
To Screen or Not To Screen, That is the Question
Featured Article
US Preventive Services Task Force. 2023. Screening for Skin Cancer US Preventive Services Task Force Recommendation Statement. JAMA 329 (15): 1290–1295.
Introduction
Skin cancer, a collection of diseases including melanoma and non-melanoma skin cancer (NMSC), is the most common human malignancy. In 2012, it was estimated that over 3 million people in the United States (U.S.) were treated for NMSC, and that there were over 5 million total NMSCs treated in the country1. Separately, the U.S. National Cancer Institute (NCI) reports melanoma is the fifth most common cancer in the United States1, constituting around 5% of newly diagnosed cancers2. The rate of new melanoma cases has risen from 17.5 cases per 100,000 persons in 1999 to 24.1 cases in 20192, with studies suggesting that the increased number of cases may be associated with increased screenings3,4. Despite the increased number of cases diagnosed, the number of deaths from melanoma annually has decreased over the past decade to just under 8,000 in 20202.
1 Of note, basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (CSCC) are not cataloged in the NCI’s Surveillance, Epidemiology, and End Results (SEER) Program. Therefore, total estimates of human cancer are underestimated. Accordingly, melanoma is likely the seventh most common cancer, behind BCC, CSCC, breast, prostate, lung and colorectal.
This decrease in death may be related to a number factors. Most importantly perhaps, there have been numerous advances in systemic treatments available for skin cancer over the past decade. Multiple checkpoint immunotherapies and targeted therapies for specific oncogene mutations have been developed to treat advanced melanoma5–7 and NMSC8–14.
However, one area that has received less attention, with less robust data, is research on the efficacy of skin cancer screenings. Intuitively, early detection should be beneficial and potentially has contributed to decreased deaths as well. However, current data have not shown a definitive impact, a fact that is reflected in this year’s updated The U.S. Preventive Services Task Force (USPSTF) guidelines.
Background on USPSTF
The USPSTF was founded in 1984 as a volunteer group of experts who would issue evidence-based recommendations on preventive healthcare services for asymptomatic patients15. The recommendations are ranked “A”, “B”, “C” or “D” based on the strength of evidence for them, with an “I” recommendation indicating that there is insufficient evidence (Table 1)15. The USPSTF initially gave skin cancer screenings a “C” recommendation in 1996 and stated there was “insufficient evidence to recommend for or against routine screening for skin cancer by primary care providers using total-body skin examination”16.
The 2023 USPSTF “concludes that the current evidence is insufficient to assess the balance of benefits and harms of visual skin examination by a clinician to screen for skin cancer in adolescents and adults”17. This “I” recommendation incorporates seventeen new studies. Overall, the USPSTF considered research from three studies on the benefits of skin cancer screening (n=NR in one study; 1,791,615 in the other two), two studies on the drawbacks of skin cancer screening (n=232), six studies on the association between routine clinician skin examination and stage or lesion thickness at skin cancer detection (n=2,947,595), and nine studies on the association between stage at skin cancer detection and melanoma or all-cause mortality (n=1,326,051)17. These studies highlighted the lack of data on the benefits of screenings.
Current research on skin cancer screenings has shown some potential harms of screenings. Screenings require significant utilization of healthcare resources, with one study finding that it costs approximately $32,000 to diagnose one melanoma and around $2,500 to diagnose one NMSC18 when screening is performed by full body skin examinations in a dermatology office. In addition, full total-body skin examinations (TBSE) are time-consuming. Per one analysis, it would require, on average, 4.5 hours of face-to-face time for a dermatologist to diagnose one skin cancer via TBSE in adult patients that presented to clinic for a focused complaint19. Extrapolating from their data, it would take 38 hours to detect a melanoma20. The time that dermatologists spend doing total-body skin examinations in populations with lower risk for serious skin disease can limit access for patients with higher risk. This poses an intriguing question: When dermatologists have limited time to screen patients and when there is a long wait for a skin screening, which populations need the most attention?
Multiple studies have suggested that limited access to timely healthcare contributes to the increased incidence of advanced melanomas at diagnosis and decreased survival in nonwhite populations21–23. A cross-sectional audit study of wait times for dermatology clinic appointments in California showed that there was no significant difference in wait times for patients who had Medicaid vs. Anthem BC, a private insurance24. However, residents in the more rural Central Valley had less access to skin cancer screenings at dermatology or family medicine practices compared to residents in the San Francisco Bay Area due to increased wait times and low rates of Medicaid acceptance, which was especially relevant given the higher portion of Central Valley residents on Medicaid24. A qualitative analysis of Latinx and non-Latinx white adults in the state also revealed that difficulties in receiving dermatology referrals and long wait times were some of the barriers to healthcare in this population25.
Furthermore, an official diagnosis of an early stage or easily treatable skin cancer, such as basal cell carcinoma or melanoma in situ, can have repercussions for patients on life insurance. Patients have reported that their polices have increased after diagnosis in spite of the efficacy of current treatments to cure these lower-risk cancers. Indeed, one study observed that patients with melanoma in situ lived longer than the general population, even though these patients still had a slight increased risk of melanoma-specific mortality26.
In addition, research has not shown that screening decreases mortality. A study done in Schleswig-Holstein, Germany in people starting at age 35 showed that there was no significant difference in melanoma mortality five years later in people who were screened compared to baseline27. In particular, screenings may not detect higher-risk melanomas, which are associated with increased mortality. A study found no difference when it compared the incidence rate of thick melanomas >1mm and >4mm in populations with melanoma screening and without28.
Journal Club
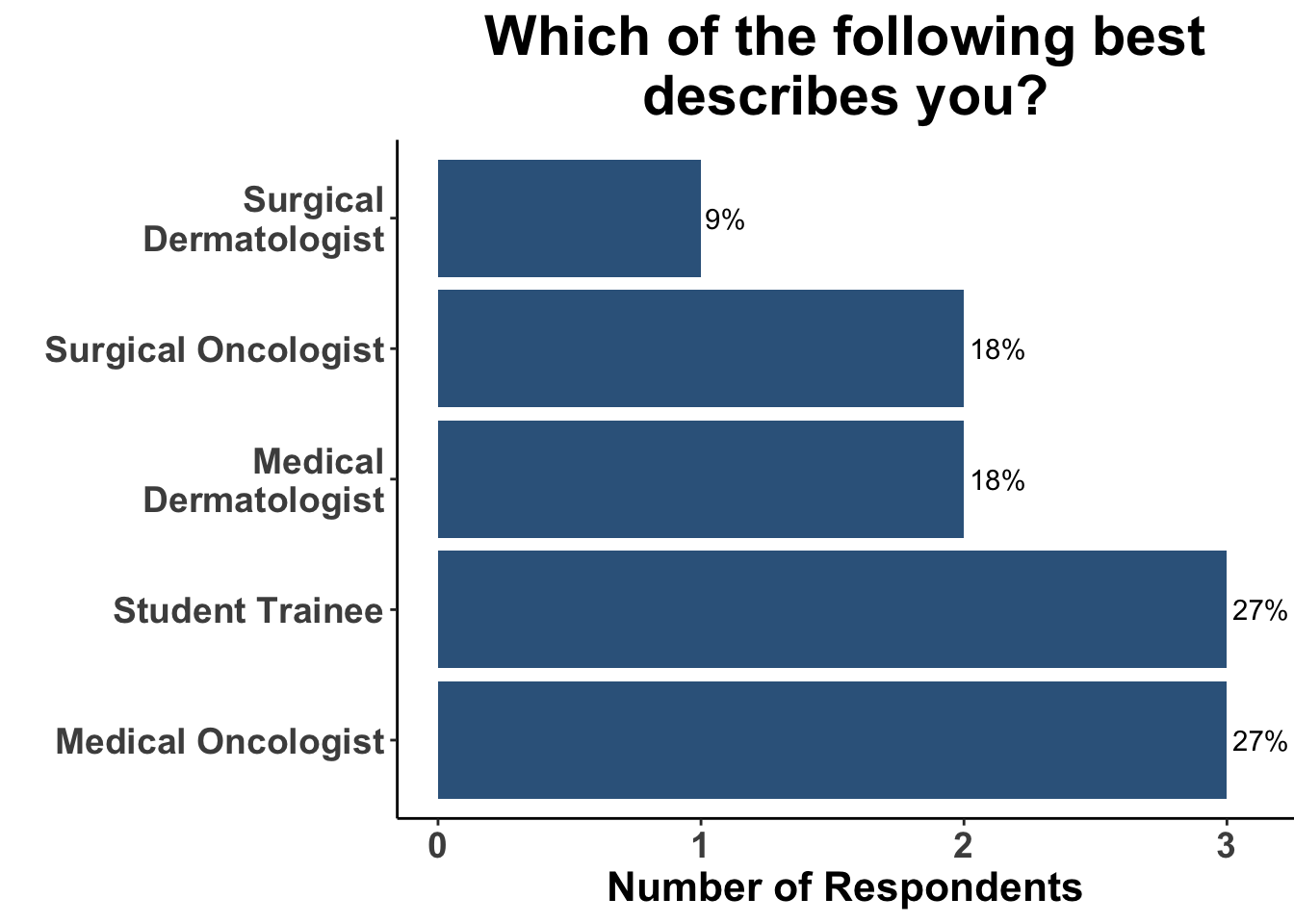
On June 9, 2023, the multi-institutional Society of Cutaneous Oncology (SoCO) Journal Club (JC) reviewed the most recent USPSTF recommendations for skin cancer screening. Participants included clinicians and investigators (Figure 1) from Massachusetts General Hospital, Massachusetts Eye and Ear, Brigham and Women’s Hospital, University of Washington, Moffitt Cancer Center, National Institutes of Health, and the University of Pittsburgh Medical Center. This Perspectives on the Science article reflects the views of the authors after the Journal Club. Please note that it does not represent the views of any other members of SoCO or affiliated institutions. In this piece, we provide a multi-disciplinary perspective on USPSTF recommendations.
Importance of Identifying Appropriate Population for Screening
Identifying individuals that will have a net benefit of a medical intervention is a critical parameter in all of healthcare and is a particular focus in USPSTF recommendation statements. In cancers with either moderate or substantial net benefit of screening (e.g. colorectal, breast and lung), specific at-risk features have been incorporated into their respective recommendation statements. For example, for colorectal cancer screening, the USPSTF has deemed that there is a high certainty of substantial net benefit (Grade “A”) for screening in all adults aged 50 to 75 years, while a high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial (Grade “B”) for adults aged 45 to 50 years29. In contrast, the USPSTF concluded that there was at least moderate certainty that the net benefit is small (Grade “C”) in screening adults aged 76 to 85 years.
For skin cancer screening, the USPSTF Grade “I” recommendation applies to asymptomatic adolescents and adults, as there is a lack of data in more nuanced, potentially at-risk populations. Thus, clinicians may incorporate previously identified risk factors for skin cancer (e.g. sun-sensitive phenotypes or family history of skin cancer30) to guide their screening practices (Table 2). Nevertheless, developing a expert consensus on which demographics are most likely to benefit from skin cancer screening would provide much needed guidance and standardization for practicing physicians.
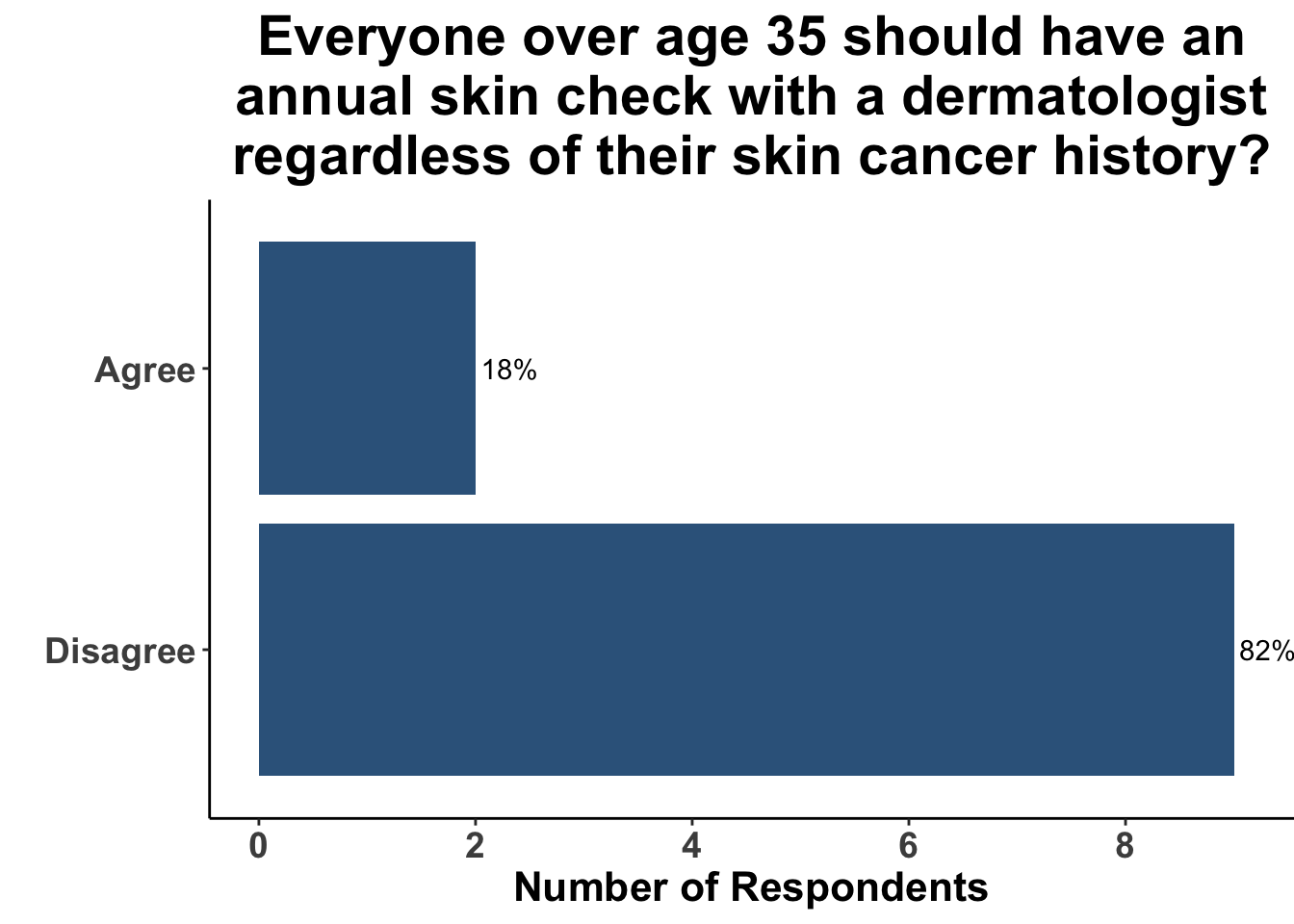
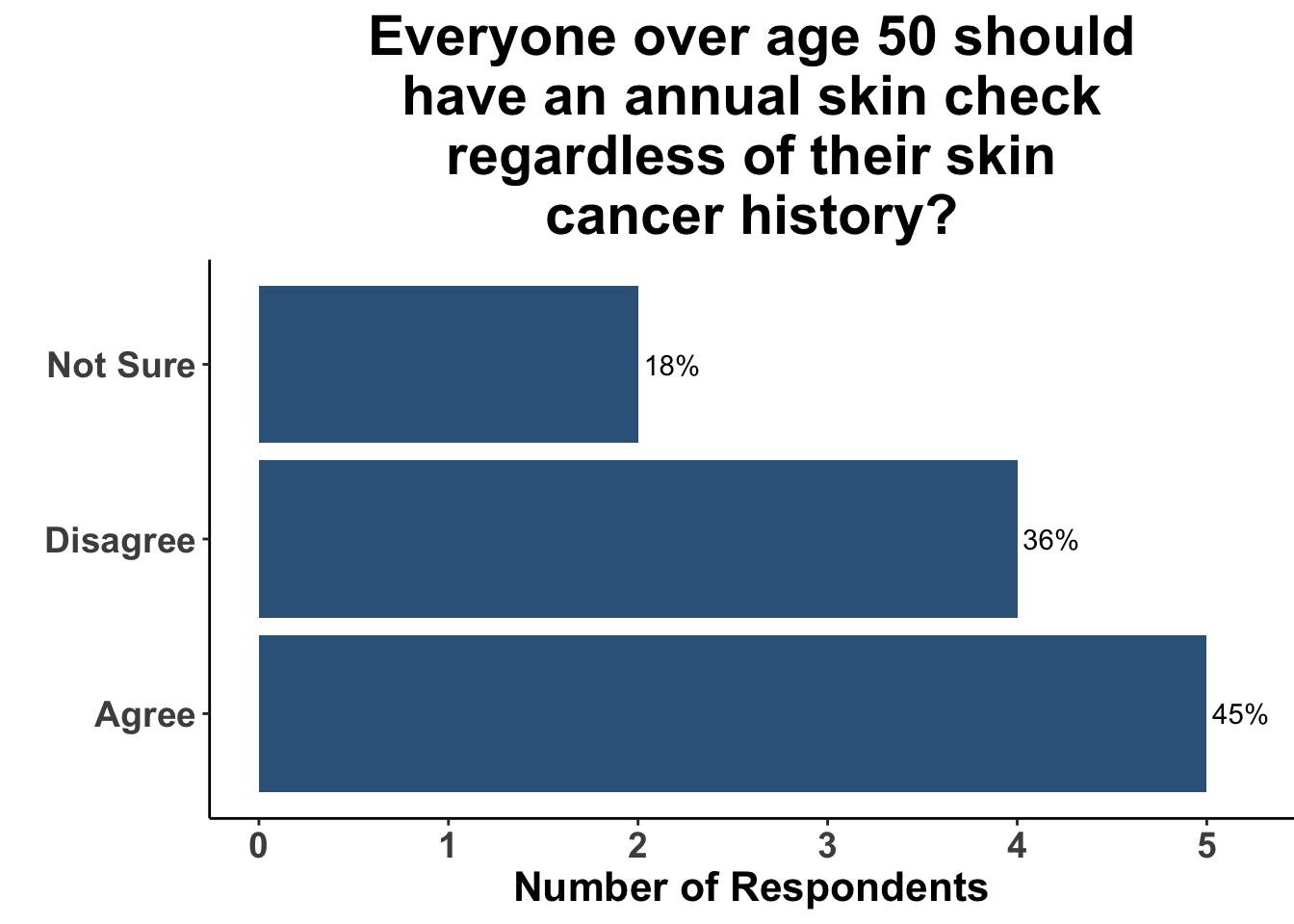
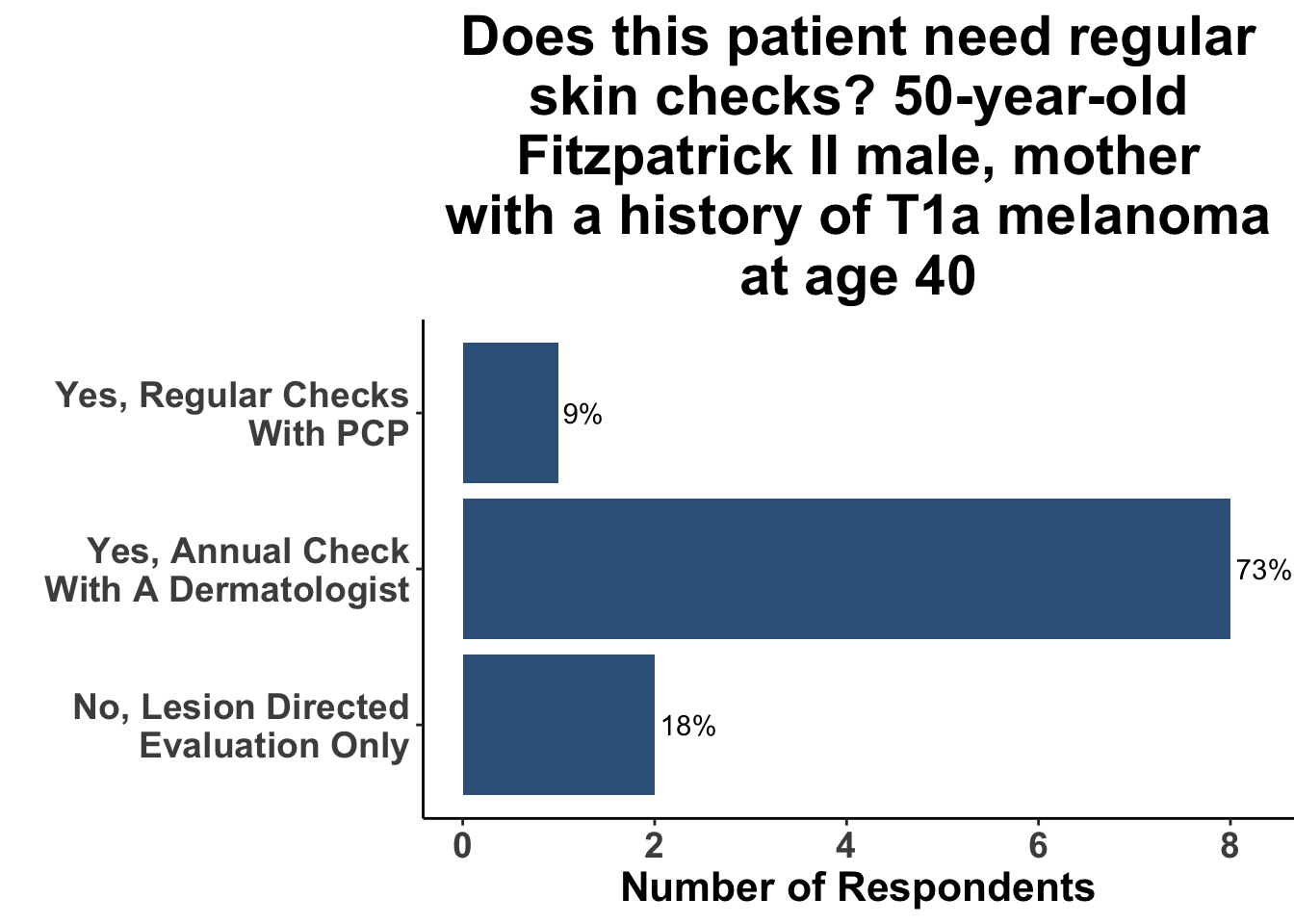
A survey at the June 9th SoCO Journal Club highlights the wide variation in approach to skin cancer screening at present. While there was general agreement that all patients over the age of 35 years do not warrant an annual skin check with a dermatologist regardless of skin cancer history (Figure 2), the group was more divided when the hypothetical patient was over 50 (Figure 3). That said, only a minority recommended skin cancer screening in both of these scenarios.
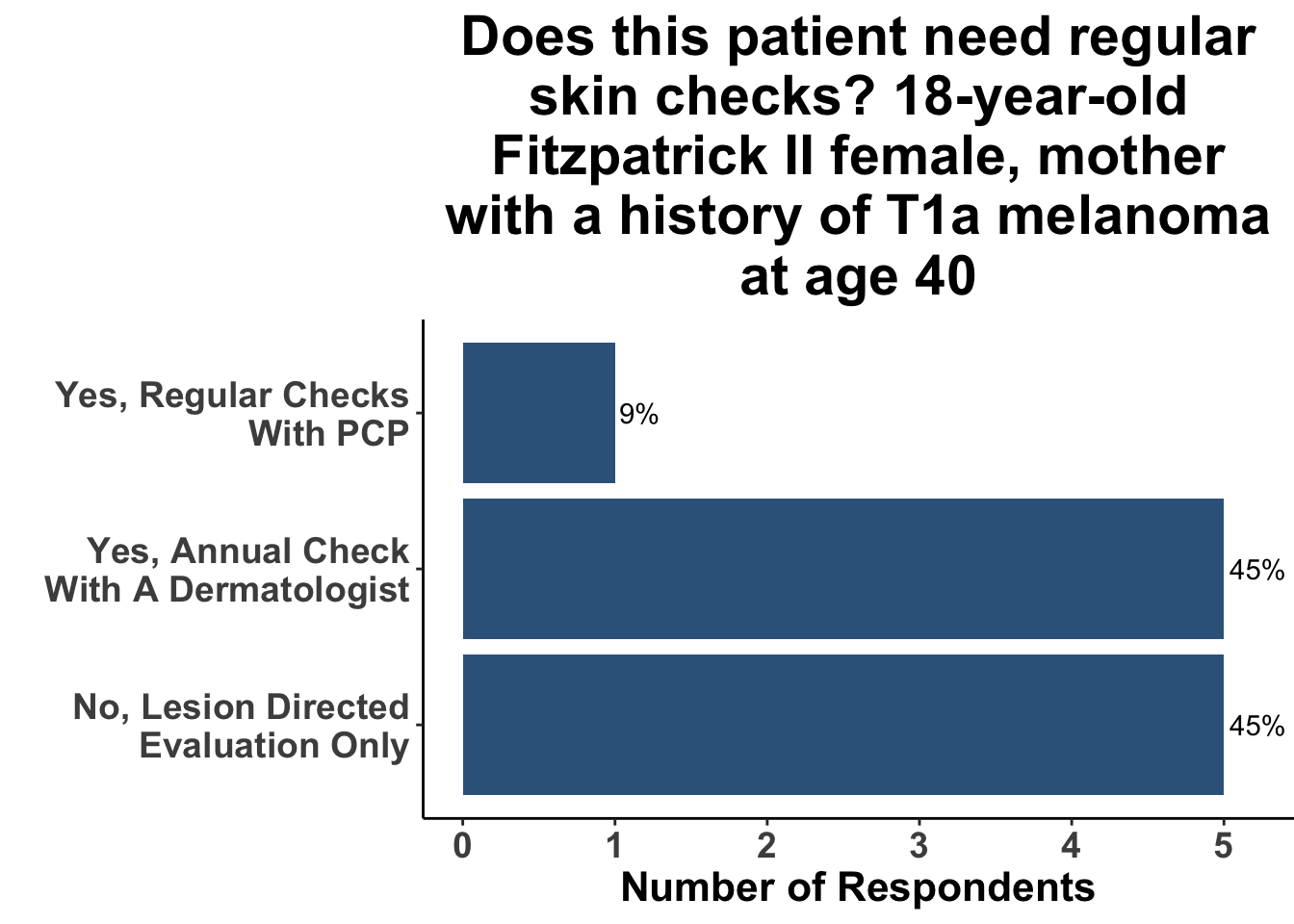
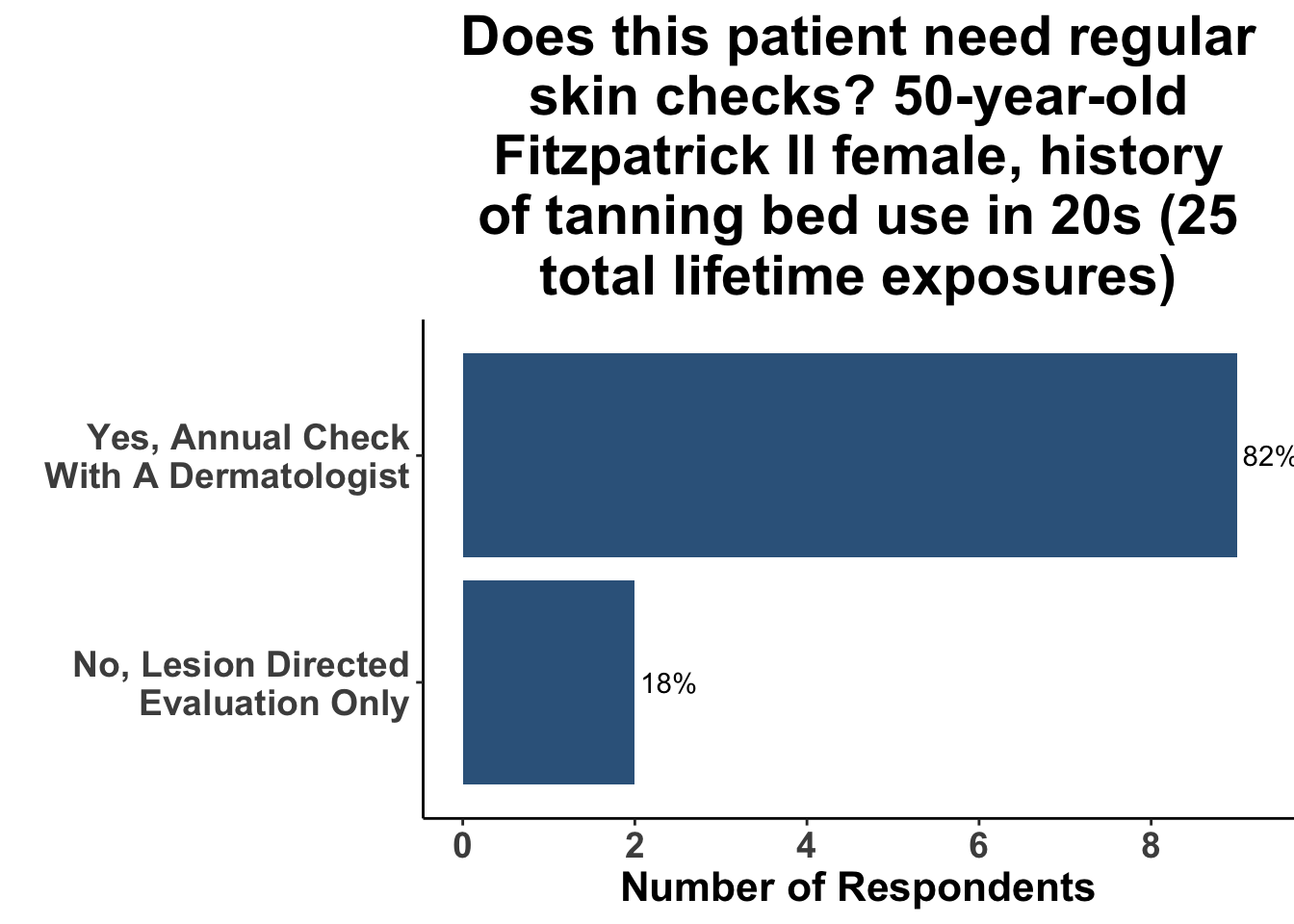
In contrast, when patients had risk factors such as a lighter skin type, history of tanning bed use, or family history of skin cancer, a greater proportion of Journal Club participants agreed that screening was indicated, although there was a wide variation in agreement (Figures 4-6).
These examples underscore the critical importance of providing high-quality data on the potential benefits of screening patients, risk-stratified by phenotype, and ideally, genotype, as well. While observational studies can provide important data on associations of skin cancer screening and outcomes, they have important limitations that affect inferences regarding causality. For example, a retrospective observational study of a screening initiative promoting annual full body skin exams in the University of Pittsburgh healthcare system was limited by a non-random sample and physicians documented screenings31. In addition, inferences made on survival after a diagnosis can be confounded by both length-time and lead-time bias32. Separately, but also importantly, studies that rely on surrogate outcomes of morbidity and mortality, such as tumor thickness, can lead to overdiagnosis 2.
2 Overdiagnosis is a result of length-biased sampling and arises when a histological diagnosis of a cancer that is unlikely to result in mortality is made. An example would include a diagnosis of early stage melanoma that did not possess the biologic activity for metastasis.
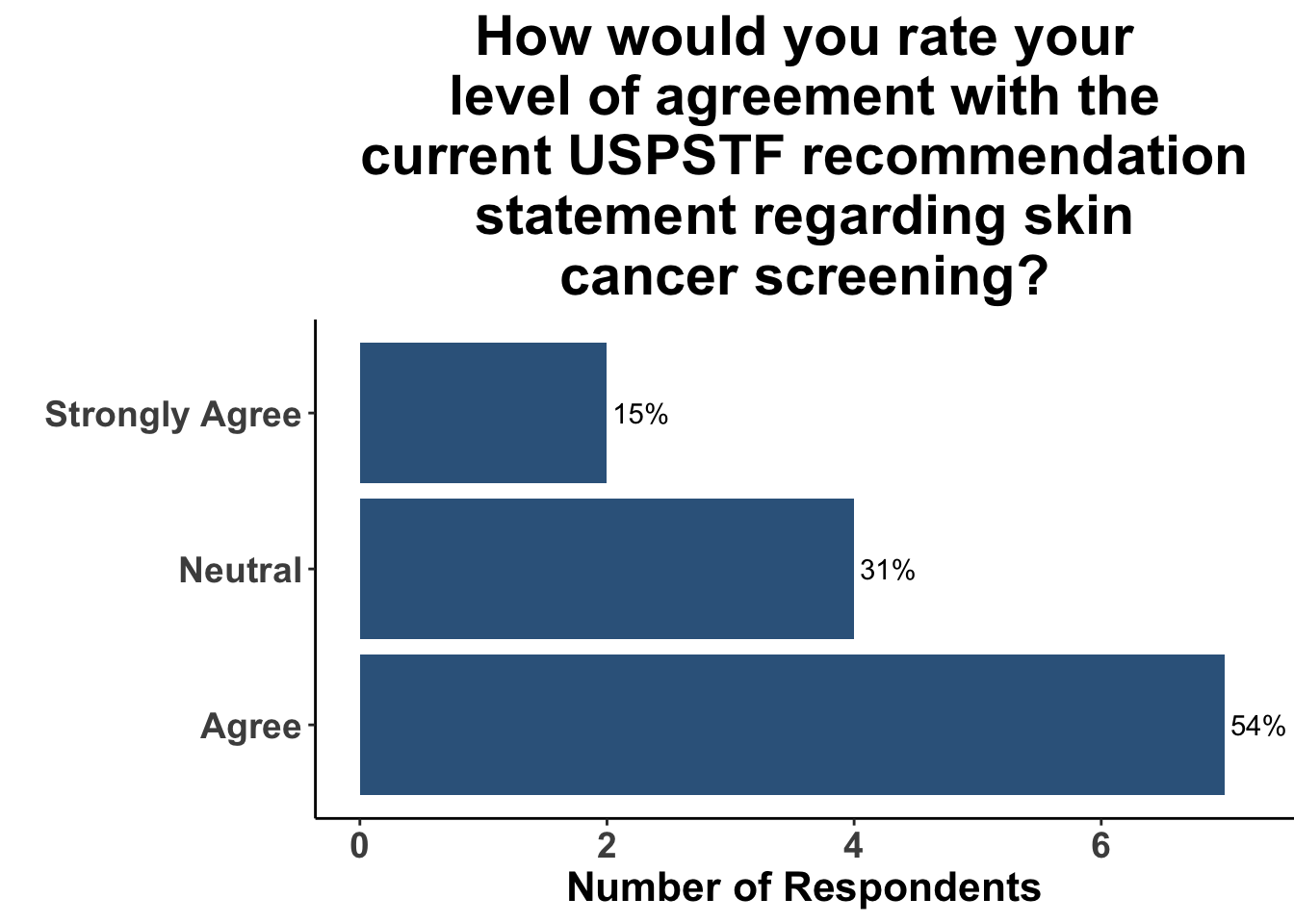
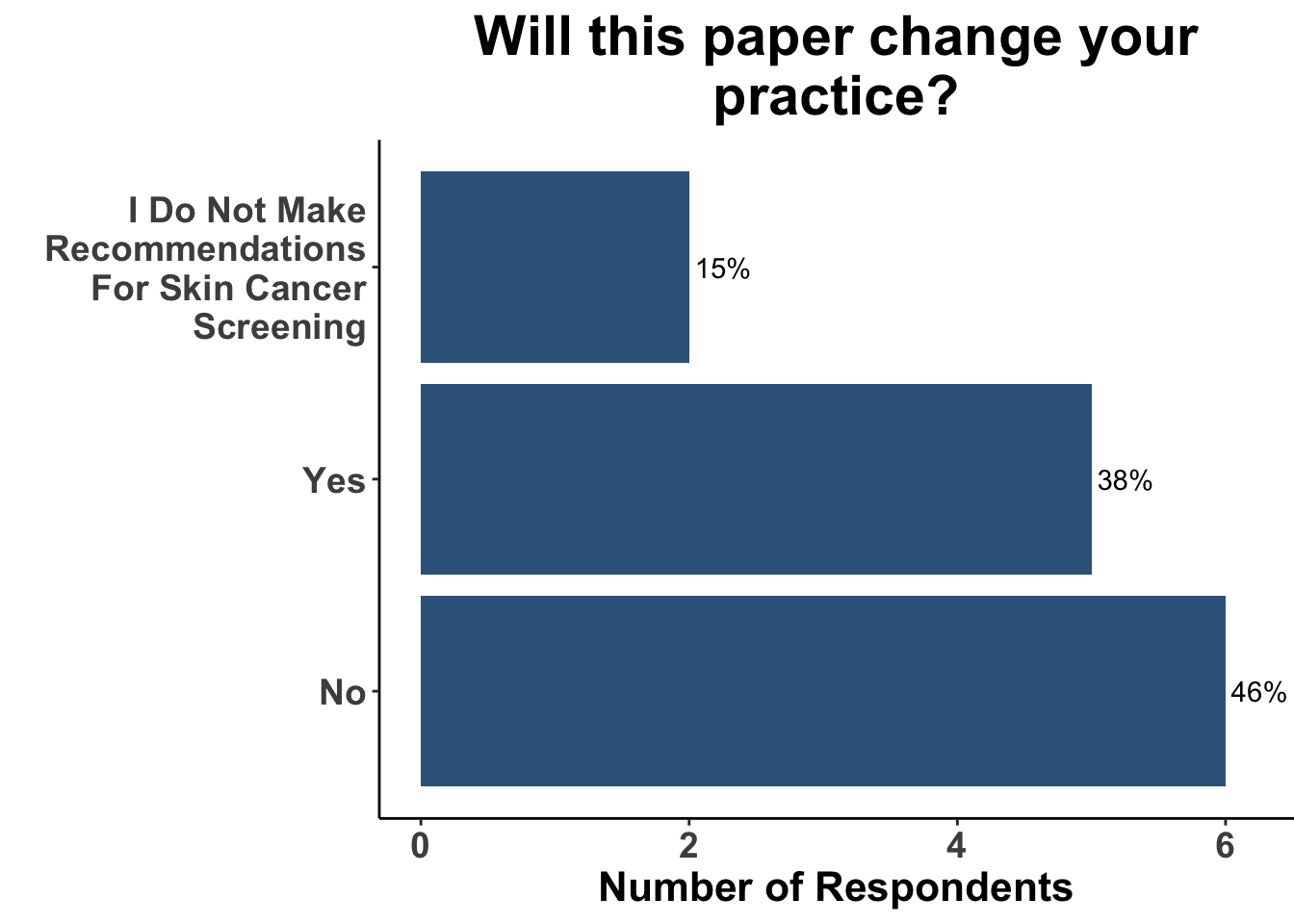
Given these limitations, randomized controlled trials (RCTs) are typically required to convincingly demonstrate evidence of a causal relationship between an intervention (e.g. skin cancer screening) and an outcome (e.g. reduction of morbidity and/or mortality from skin cancer). However, a major obstacle in acquiring data via RCTs is the logistical challenges associated with them. Most significant of these is cost. For example, a prospective randomized control trial of melanoma screening in Australia cost $8.4 million dollars33. Preliminary results from the trial highlighted the feasibility of implementing local screening initiatives that garner broad community support and engagement34. However, it only received funding for around half of the cost, and the trial was never completed due to lack of funding33. Thus, it is seems unlikely that the next USPSTF statement in regards to skin cancer screening will achieve an “A” or “B” recommendation. Given the lack of high-quality data currently available, over two-thirds of SoCO JC attendees agreed with the current “I” recommendation (Figure 7). Furthermore, since the the 2023 recommendation statement did not substantively change from their 2016 recommendation, only a minority of participants replied that this recent update would change their practice (Figure 8).
Recent Contributions to the Skin Cancer Screening Conversation
Not surprisingly, the updated USPSTF recommendation statement has engendered additional thoughtful perspectives on skin cancer screening. Recent editorials have emphasized that the continued “I” grade represents an opportunity for larger, more focused studies consisting of racially and ethnically diverse groups of people at higher risk for skin cancers. Drs. Adamson35 and Asgari36 stressed the importance of determining who would benefit most from screening, potentially based on clinical and environmental risk factors and/or polygenic risk scores from genome-wide association studies. In particular, Dr. Adamson35 called for research to better characterize the natural history of early melanoma to optimize screening and treatment for people with melanomas at a higher risk of disease progression. Additionally, Drs. Adamson35 and Asgari36 highlighted the need for future studies to more precisely determine the harms of skin cancer screening, such as overdiagnosis and psychosocial harms.
In order to foster an environment where such research could happen, Dr. Asgari36 advocated for the USPSTF Annual Report to Congress on High-Priority Evidence Gaps for Clinical Preventive Services to include skin cancer screenings as an evidence gap. They also suggested that the National Institutes of Health could categorize a skin cancer screening trial as a highest priority challenge topic36.
Summary
In 2023, the USPSTF has given skin cancer screenings an “I” recommendation, stating that there is insufficient evidence for the benefits or harms of skin examinations in asymptomatic adolescents or adults. This recommendation has been largely unchanged since 1996, highlighting the lack of research conclusively showing the benefits of screenings. Currently, there is some data on the potential harms of screenings, including the costs on the healthcare system, challenges with access to qualified dermatologists, and the financial impacts on patients and the health care system. However, in order to determine any benefits to skin cancer screenings in select cohort populations, costly prospective trials would be required.
Materials and Methods
This Perspectives on the Science piece was published using Quarto®. The survey was conducted using REDCap®.37 The figures depicting the survey data were created using R (version 4.0.0) and the tidyverse suite of packages,38 including ggplot2.39 The image on the “Perspectives on the Science” page was created by the authors (DMM) using the rosemary package.40
Bibliography
Appendix
Citation
@article{lee2023,
author = {Lee, Truelian and M. Miller, David and L. Kaufman, Howard
and S. Emerick, Kevin and Gupta, Sameer and K. Ferris, Laura},
publisher = {Society of Cutaneous Oncology},
title = {To {Screen} or {Not} {To} {Screen,} {That} Is the {Question}},
journal = {Journal of Cutaneous Oncology},
volume = {1},
number = {2},
date = {2023-07-30},
url = {https://themillerlab.io/publications/to_screen_or_not_to_screen},
doi = {10.59449/joco.2023.07.30},
issn = {2837-1933},
langid = {en}
}