Skin Cancer Chemoprevention for Transplant Recipients - The Search Continues
Featured Article
Nicotinamide for Skin-Cancer Chemoprevention in Transplant Recipients. Allen et al. New England Journal of Medicine 388 (9): 804–12.
Introduction
On March 6th, 2023 the multi-institutional Society of Cutaneous Oncology (SoCO) Journal Club reviewed the recently published New England Journal of Medicine article “Nicotinamide for Skin-Cancer Chemoprevention in Transplant Recipients”(Allen et al. 2023). Participants included clinicians and investigators from Massachusetts General Hospital, Mass Eye and Ear, the National Institutes of Health, George Washington Cancer Center, Stanford Medical Center and Northwestern Medical Center. Importantly, the comments in this article represent the views of the authors of this Perspectives on the Science piece after the Journal Club. It does not represent views of any other members of the SoCO or the affiliated institutions. In this article we provide a summary of the discussion regarding this important contribution to the literature.
Background for the Study
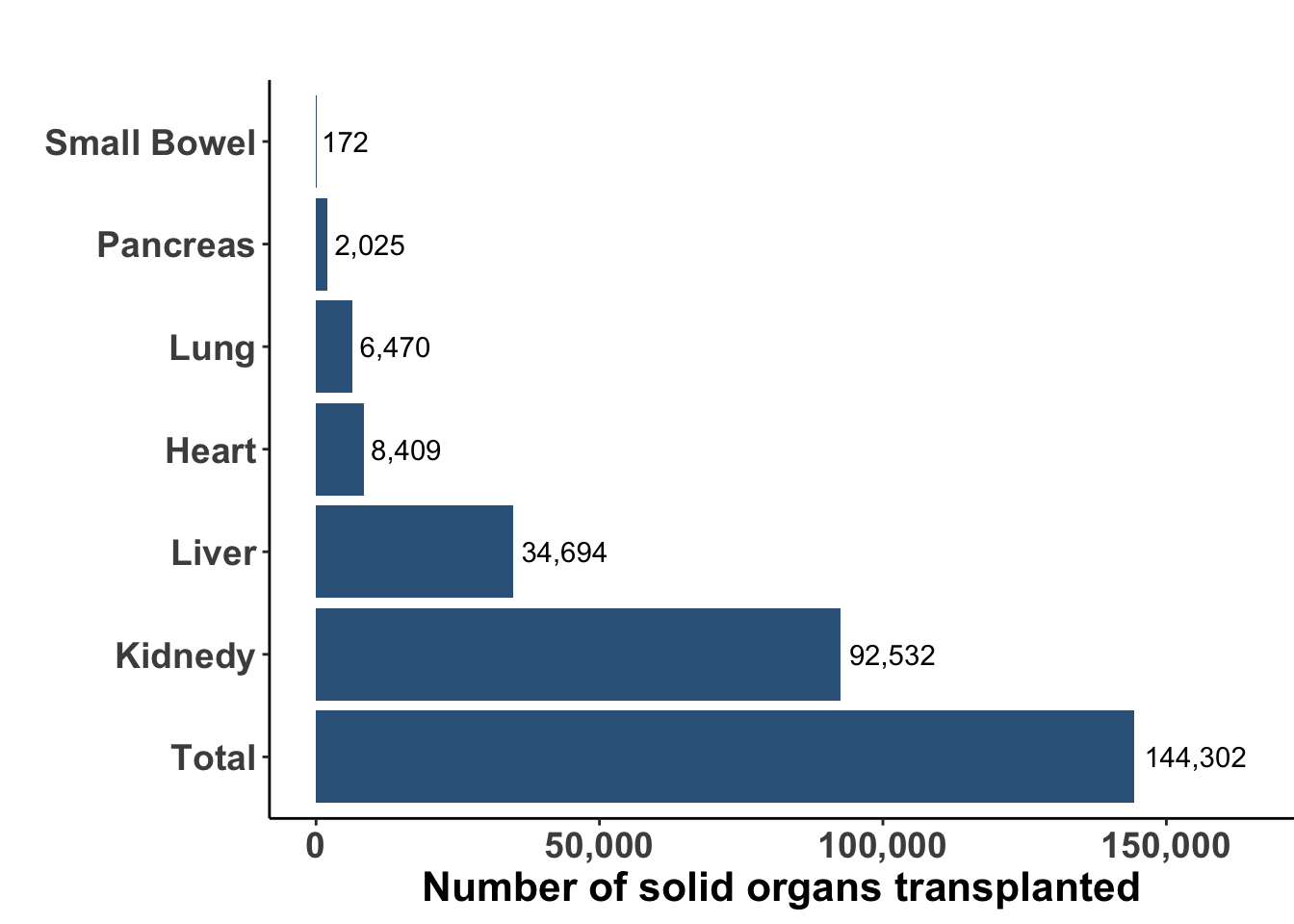
Solid organ transplantation is a life-saving procedure for patients with end-organ dysfunction or damage. While advances in both surgical technique, organ harvesting, and development of immune suppression have resulted in an increasing number of organ transplant procedures worldwide, many challenges remain such as the availability of appropriate donor allograft organs and the systemic sequelae of immune suppression. In 2021 there were an estimated 144,302 solid organ transplants performed worldwide (Figure 1) (Elflein 2023). Kidney continues to be the most commonly transplanted organ with liver and heart next in incidence. According to the United Network for Organ Sharing, there were over 42,000 solid organ transplants in the United States during 2022, an annual record number with nearly 117 transplant procedures every day. To avoid rejection, patients are placed on life-long immunosuppressive medications, which may vary depending on the organ being transplanted, the degree of HLA mismatch, and the timing of transplantation. A variety of drugs have been used alone and in combination (see Table 1).
The use of chronic immunosuppression in organ transplant recipients results in an increased risk of infections and cancer. The increased risk of cancer has been recognized for decades and in 2011 the incidence of cancer was reported to be 1375 cases per 100,000 person-years, but this was based on the review of tumor registries only (Engels et al. 2011). In this report, the risk was elevated for 32 different types of cancer, including several known to be associated with a viral etiology, such as anal carcinoma and Kaposi’s sarcoma, as well as those cancers without a known viral cause, such as melanoma, lung carcinoma and thyroid cancer. The most common cancers were non-Hodgkin lymphoma and lung cancer. While these early reports highlighted the increased risk for cancer in solid organ transplant recipients (SOTRs), the lack of reporting for non-melanoma skin cancers (NMSC) meant that keratinocyte-associated tumors were not evaluated. More recently, it has become clear that the incidence of non-melanoma skin cancers is around 50-fold higher than in immune competent individuals (Tessari and Girolomoni 2012). Current estimates suggest that 50% of all organ transplant recipients will develop a non-melanoma skin cancer at some point after transplantation, and these will most commonly be cutaneous squamous cell carcinomas (CSCCs) and basal cell carcinomas (BCCs).
Most superficial NMSCs in immune competent and organ transplant recipients are usually treated with resection - including Moh’s surgery, excision with post-operative complete circumferential peripheral and deep margin assessment (CCPDMA), and standard wide local excision with sectional assessment - and radiation therapy alone or in an the adjuvant setting. However, recurrent tumors, high risk lesions, locally advanced, and metastatic disease has largely been treated with systemic therapy, including immune checkpoint blockade (Migden et al. 2018; Gross et al. 2022; Shalhout et al. 2021, 2022; David M. Miller et al. 2022). While the success of immune checkpoint blockade for the treatment of locally advanced and metastatic keratinocyte carcinomas (KCs) has revolutionized the management of non-melanoma skin cancer in immune competent patients, the role of immunotherapy for patients with solid organ transplants is more complicated.
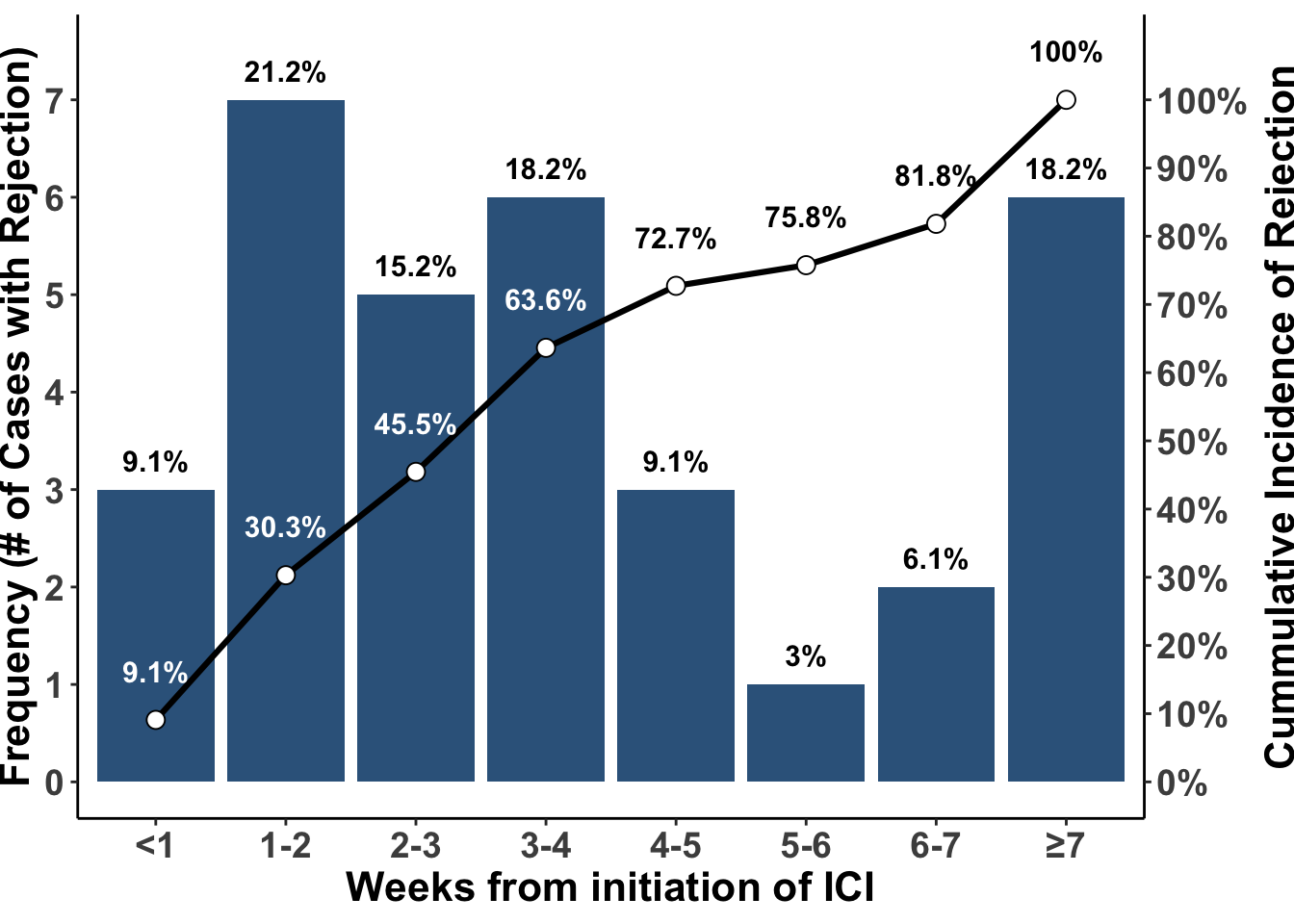
Early case reports and small clinical series have suggested a high rate of acute rejection or graft failure following the use of immune checkpoint blockade. In a recent meta-analysis, an overall acute rejection rate of 41.2% and graft failure rate of 23.5% was reported (Portuguese et al. 2022). In this study, allograft rejection was highest in renal transplant patients at 48.4%. Most of the rejection occurred in the first month of treatment and nearly all occurred within the first seven weeks of treatment (Figure 2). Although the incidence of rejection appears slightly lower in non-renal transplant recipients, the lack of an alternative source of functional organ preservation, such as through dialysis, makes immunotherapy with immune checkpoint blockade risky in liver, lung, heart, and bowel transplant recipients. Thus, prevention or early detection of skin cancer is a major priority for patients who have received a solid organ transplant.
Although there are no universally accepted preventive measures for non-melanoma skin cancer, there have been several strategies evaluated over the last decades, including behavioral modification, physical barriers, and preventative agents. Table 2 provides an overview of several of these approaches. The standard approach for skin cancer prevention includes regular skin surveillance by an experienced dermatologist and there is extensive literature supporting this approach in both immune competent and immune compromised individuals (Rojas et al. 2022). The use of sunscreen has also been controversial, but there is data supporting a reduction in new actinic keratoses, basal cell carcinoma, squamous cell carcinoma, and melanoma with routine sunscreen application in immune competent patients (Thompson, Jolley, and Marks 1993; Pols et al. 2006; Green et al. 2011). In patients with solid organ transplants, the data does support a role for sunscreen in preventing actinic keratoses and likely cutaneous squamous cell carcinoma also (Ulrich et al. 2009). Standard management of transplant patients should include referral to a dermatologist or skin cancer screening clinic and recommendations for appropriate sunscreen use.
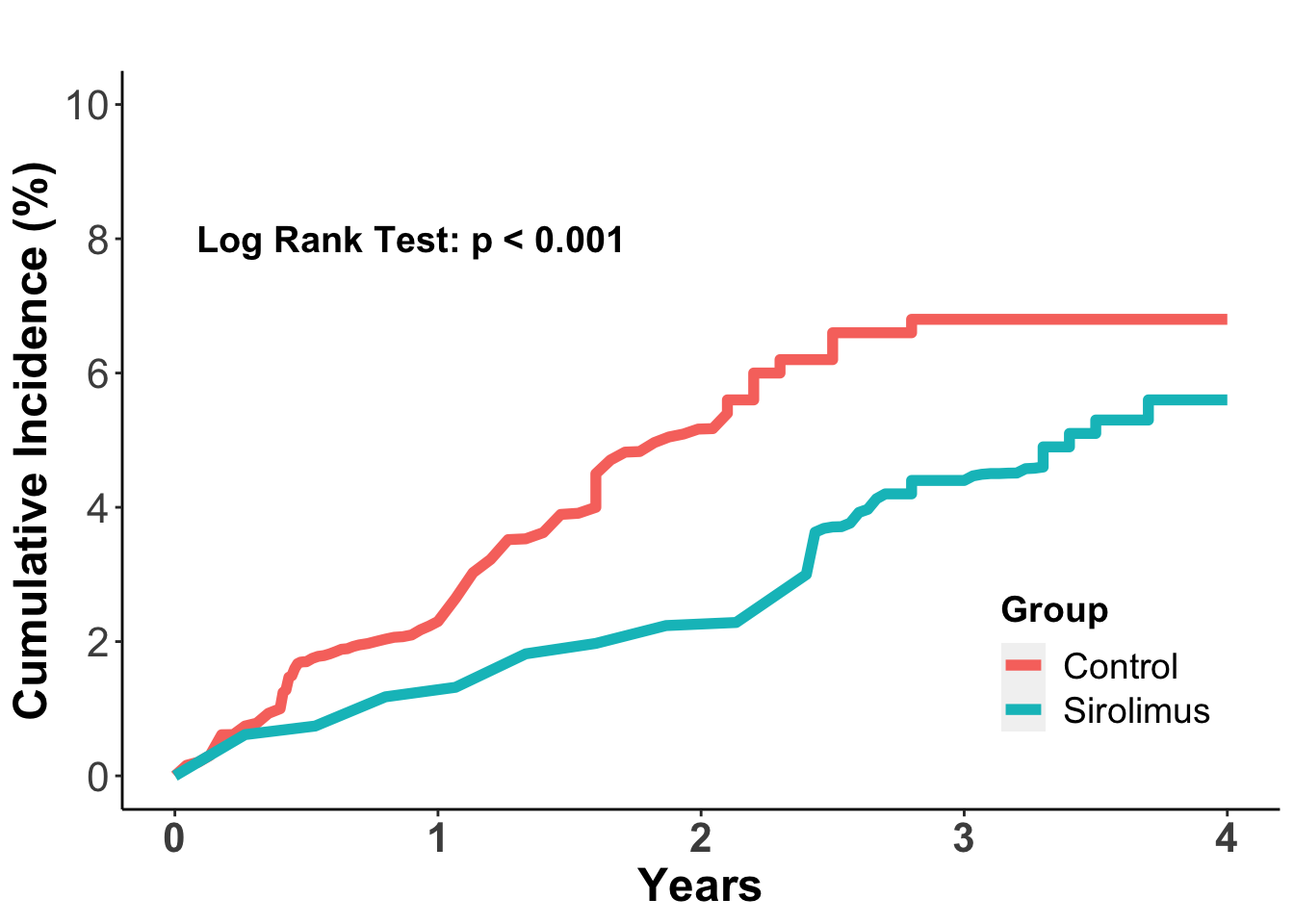
There has been some focus on the role of individual immunosuppressive agents and combination regimens in promoting potential malignant tumor initiation and progression. Although this is an area that required further investigation, there has been data suggesting that mTOR inhibitors, and in particular sirolimus, may be less likely to promote cancer progression, especially non-melanoma skin cancers. In a 2012 meta-analysis, data from 5,876 organ transplant recipients from 21 independent clinical trials in which patients were randomized to sirolimus treatment or other immunosuppressive regimens was collected for cancer incidence (Euvrard et al. 2012). In this study sirolimus treatment was associated with a 40% reduction in the risk of cancer (adjusted HR 0.60; 95% confidence interval 0.39 to 0.93). In the total population, there were 243 patients who developed a malignant tumor, which included 127 in the sirolimus group and 16 in the control group. The cumulative incidence of cancer was lower in the sirolimus group (P=0.006) (Figure 3). The effect was especially notable for non-melanoma skin cancers, where sirolimus treatment was associated with a 56% reduction in skin cancer incidence (adjusted HR 0.44, 95% confidence interval, 0.24 to 0.63) compared to non-sirolimus regimens. The effect was even more pronounced in patients who converted to sirolimus from an established immunosuppressive regimen, where there was a 68% reduction in non-melanoma skin cancers. These data have prompted a transition to sirolimus or other mTOR inhibitors once a malignancy is identified. This may not always be possible, however, and whether additional decreases in immune suppression may also help prevent skin cancer progression.
Chemoprevention approaches have included topical and oral retinoids, which are thought to prevent skin cancer through a multifactorial mechanism, which may involve improved differentiation of normal keratinocytes, induction of tumor cell apoptosis and cell death, and re-programming of host anti-tumor immunity (Ramchatesingh et al. 2022). There has been a reduction in the incidence of multiple non-melanoma skin cancers, including keratinocyte-associated tumors with retinoid administration, but these agents are not currently approved by the FDA. A major issue has been the need for high doses of oral agents, which are often associated with significant toxicity. The role of retinoids in skin cancer prevention for organ transplant recipients is more controversial, but there is some data suggesting there may be a reduction in the incidence of new cutaneous squamous cell carcinomas (Bavinck et al. 1995; George et al. 2002; Nemer and Council 2019). Despite initial efficacy, a rebound phenomenon upon treatment cessation has been observed, confounding the overall assessment of the role of retinoids as chemoprevention.
Nicotinamide is a water-soluble form of vitamin B3 (niacin) and is found in food and has been used as a dietary supplement and as a medication. Nicotinamide is a cofactor in the nicotinamide adenine dinucleotide (NADH/NAD+) complex, which is involved in numerous enzymatic oxidation-reduction reactions in cells, most notably glycolysis, the citric acid cycle, and the electron transport chain. NAD+ acts as an electron carrier that acts to covert nutrients into cellular energy in the form of adenosine triphosphate (ATP). As such, nicotinamide prevents depletion of ATP and this allows for DNA repair in ultraviolet radiation-induced damage. Nicotinamide is used as a supplement for niacin deficiency in pellagra and has been used in a cream formulation for acne treatment. Oral nicotinamide at doses of 500 – 1000 milligrams daily has been shown to reduce the rate of new actinic keratoses and non-melanoma skin cancers in high-risk, immune competent, patients (Andrew C. Chen et al. 2015). Nicotinamide is generally well tolerated, although hepatic toxicity has been reported at higher doses (Knip et al. 2000).
The role of nicotinamide for skin cancer prevention in immunocompetent subjects was evaluated in the ONTRAC trial, a prospective, double-blind, randomized, placebo-controlled phase 3 clinical trial (Andrew C. Chen et al. 2015). In this study, 386 high-risk patients, defined as having at least two non-melanoma skin cancers in the prior 5 years, were randomized to either nicotinamide given at 500 milligrams twice daily or placebo for one year. All subjects were seen by a dermatologist every 3 months for 18 months and the primary endpoint was the number of new keratinocyte tumors during the 12-month intervention period. Safety was also assessed as a secondary endpoint. The authors reported that the rate of new non-melanoma skin cancers was 23% lower in the nicotinamide group compared to patients treated with placebo (p=0.02). A similar impact was seen for both basal cell carcinomas (20% risk reduction) and squamous cell carcinomas (30% risk reduction). The number of new actinic keratoses was 13% lower in the nicotinamide group at 12 months (p=0.001). The incidence of adverse events was similar in both treatment groups. The role of nicotinamide for skin cancer prevention in solid organ recipients is not well established.
In this report, Allen and colleagues conducted a phase 3, multi-center, double-blind, randomized, placebo-controlled trial to assess the role of oral nicotinamide in the chemoprevention of keratinocyte cancers in solid organ transplant recipients. The trial, termed the “Oral Nicotinamide to Reduce Actinic Cancer after Transplant” (or ONTRANS) study was reported in the New England Journal of Medicine.
Study Design
ONTRANS was a phase 3, randomized controlled trial (Figure 4). The investigators tested the hypothesis that administration of 500 mg of nicotinamide twice daily would decrease the development of new keratinocyte carcinomas compared to placebo. The primary endpoint in this study was the number of new keratinocyte cancers during the 12-month intervention period. Based on a previous study involving kidney-transplant recipients (A. C. Chen et al. 2016), in which a mean of 4.2 KCs per participant developed within 6 months in the placebo group, the investigators hypothesized that the number of new KCs developed in 12 months in the control group would be seven. Assuming a 30% difference in the KC count at 12 months between the treatment and control group, the investigators projected that enrollment of 254 subjects would provide the trial with 80% power to test the primary endpoint with a two-sided alpha level of 5%. Key secondary endpoints included: 1) the number of new CSCCs and BCCs at 12 months, 2) squamous-cell and basal-cell carcinoma histologic subtypes and differentiation, 3) the number of keratinocyte cancers across different transplant types, 4) the number of recurrent keratinocyte cancers at 12 months, 5) the number of actinic keratoses at month six, 6) quality of life at month twelve, 7) safety and 8) drug levels of routinely measured immunosuppressants such as calcineurin and mTOR inhibitors.
Figure 4. Study Schema.
flowchart LR
classDef leaf1 fill:#E5BDE7
ONTRANS{{ONTRANS <br> SOTRs with >= 2 KCs <br> n = 158}}
ONTRANS--Treatment<br>Group--> nicotinamide(Nicotinamide 500 mg <br>BID x 12 months<br> n = 79)
ONTRANS--Control<br>Group--> placebo(Placebo x 12 months <br> n = 79)
primaryEndpoint[<b>Primary Endpoint</b> - Number of new KCs at 12 months]:::leaf1
Study Results
The study was conducted from May 2017 to August 2019. 158 subjects were enrolled from the 288 participants who were screened for eligibility. The trial was stopped early owing to poor recruitment. The mean number of new KCs during the 12-month intervention period was not different between the two groups, with subjects who received nicotinamide developing a mean of 2.6 ± 3.2 lesions compared with 2.7 ± 3.4 lesions in the placebo group (rate ratio of 1, 95% confidence interval (CI), 0.8-1.3, p = 0.96). Additionally, the number of new BCCs and CSCCs did not differ between the two groups, with rate ratios of 1.4 (95% CI, 0.8-2.3) and 0.9 (95% CI, 0.6-1.2), respectively. There were no new safety signals for the nicotinamide group.
Discussion
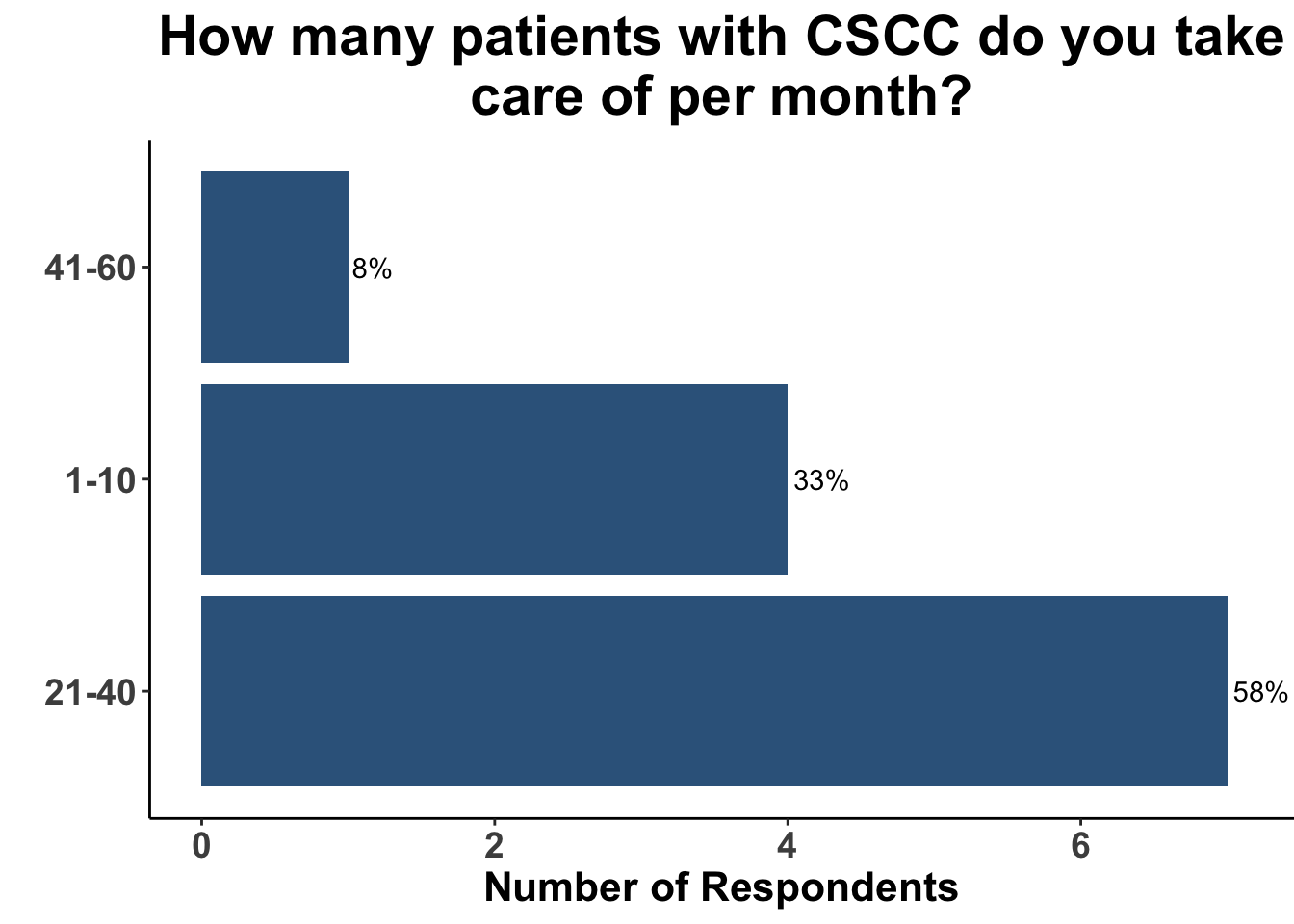
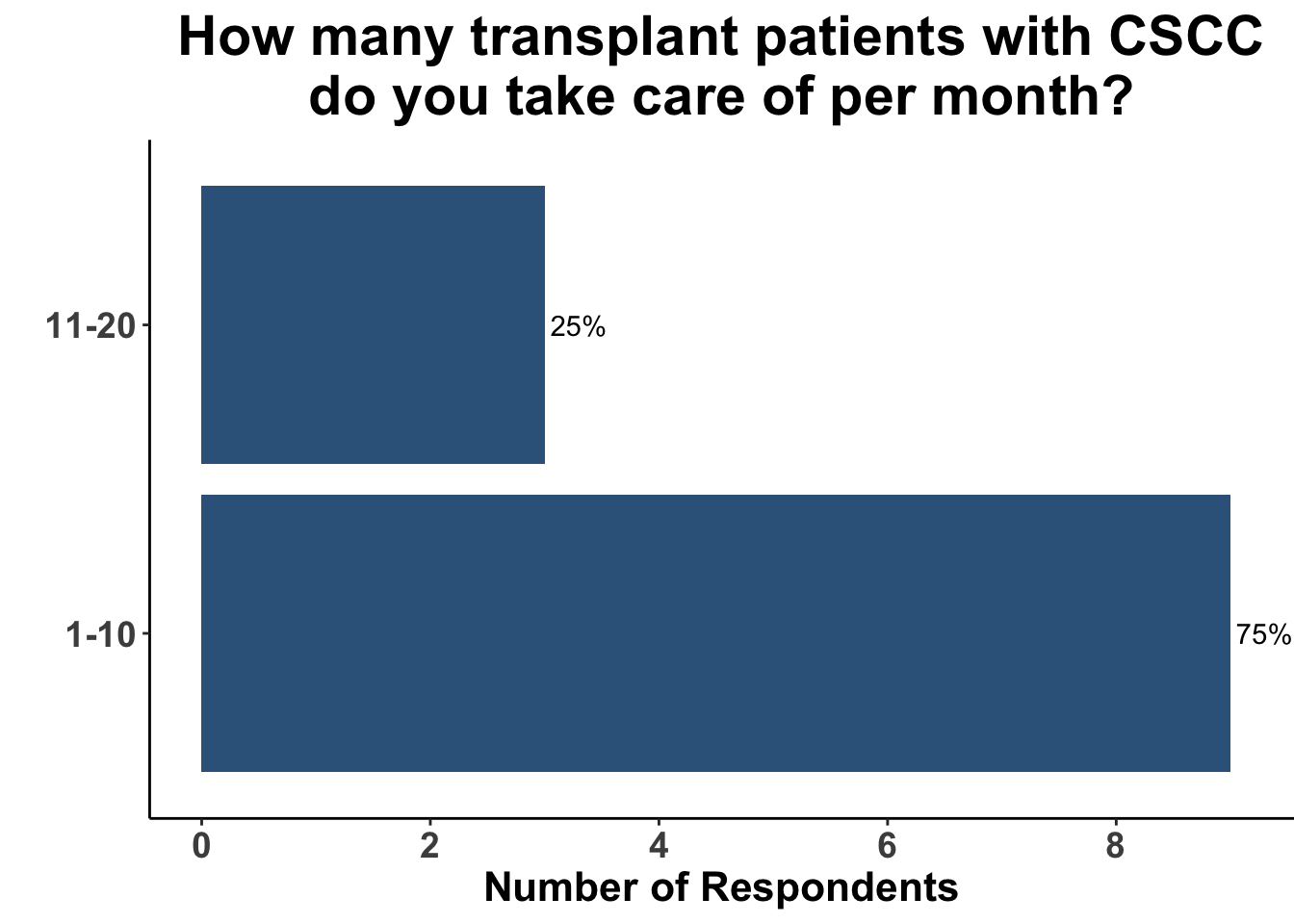
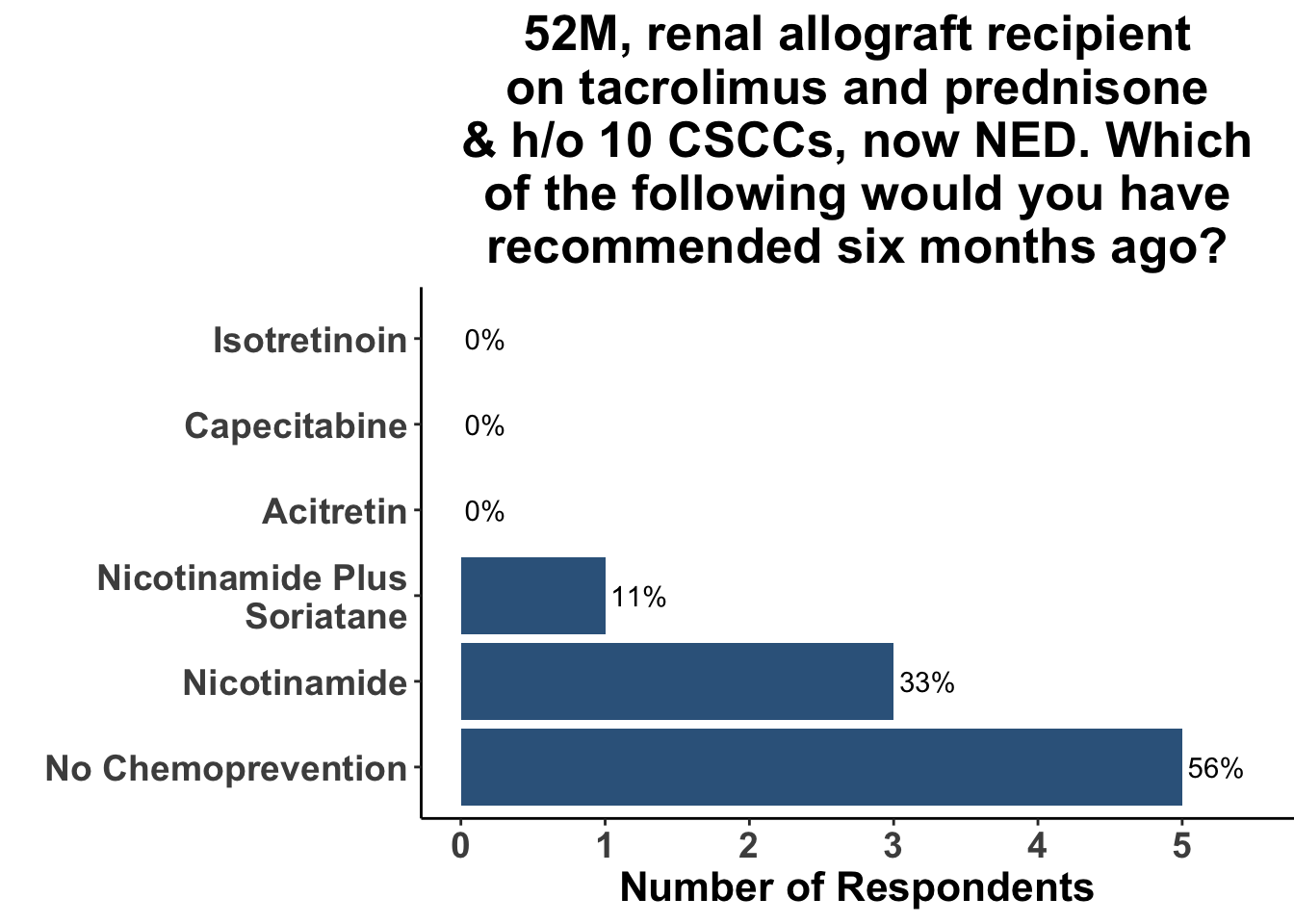
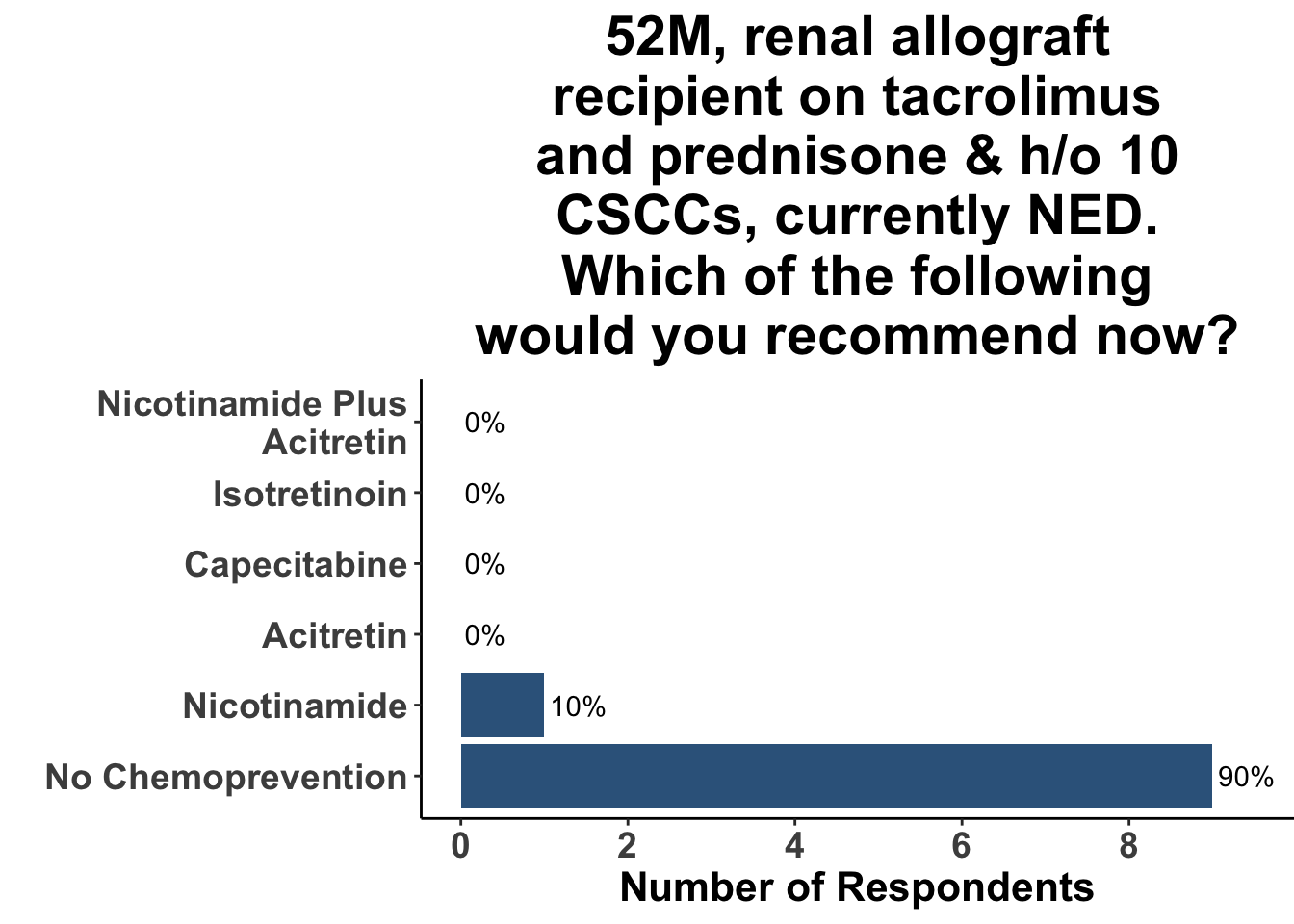
Keratinocyte carcinomas, especially CSCCs, remain a significant cause of morbidity and mortality for recipients of solid organ transplants. Unfortunately, there is a limited body of data supporting oral chemoprevention strategies, which has precluded the adoption of a standard of care for SOTRs. Interestingly, when queried on what chemoprevention strategy they would have used six months ago to manage a high-risk, renal allograft transplant patient (i.e before the publication of the ONTRANS study), the majority of the experienced SoCO practitioners who attended the March 6th Journal Club (Figures 5-7) actually replied that they would not use a chemoprevention strategy (Figure 8).
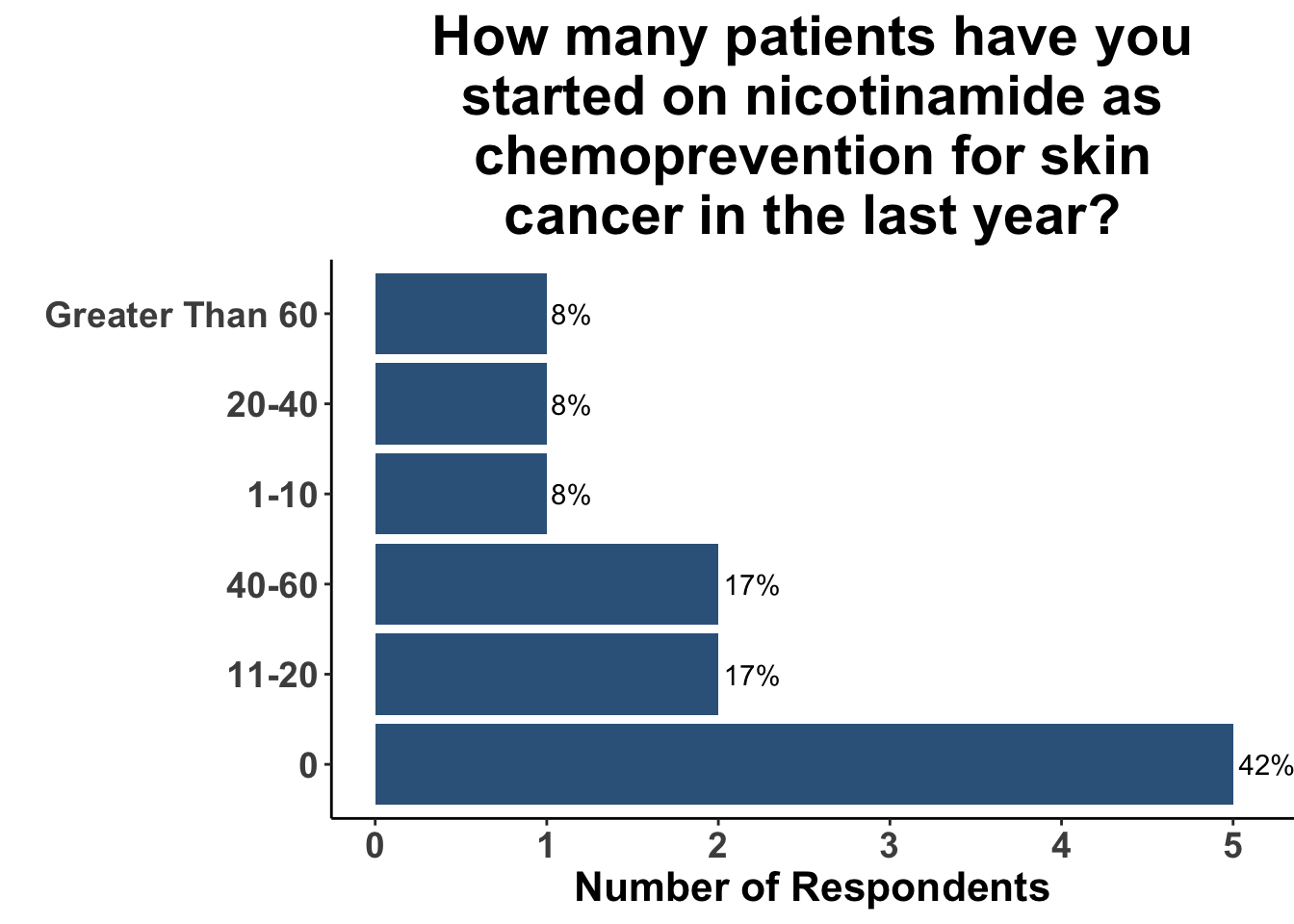
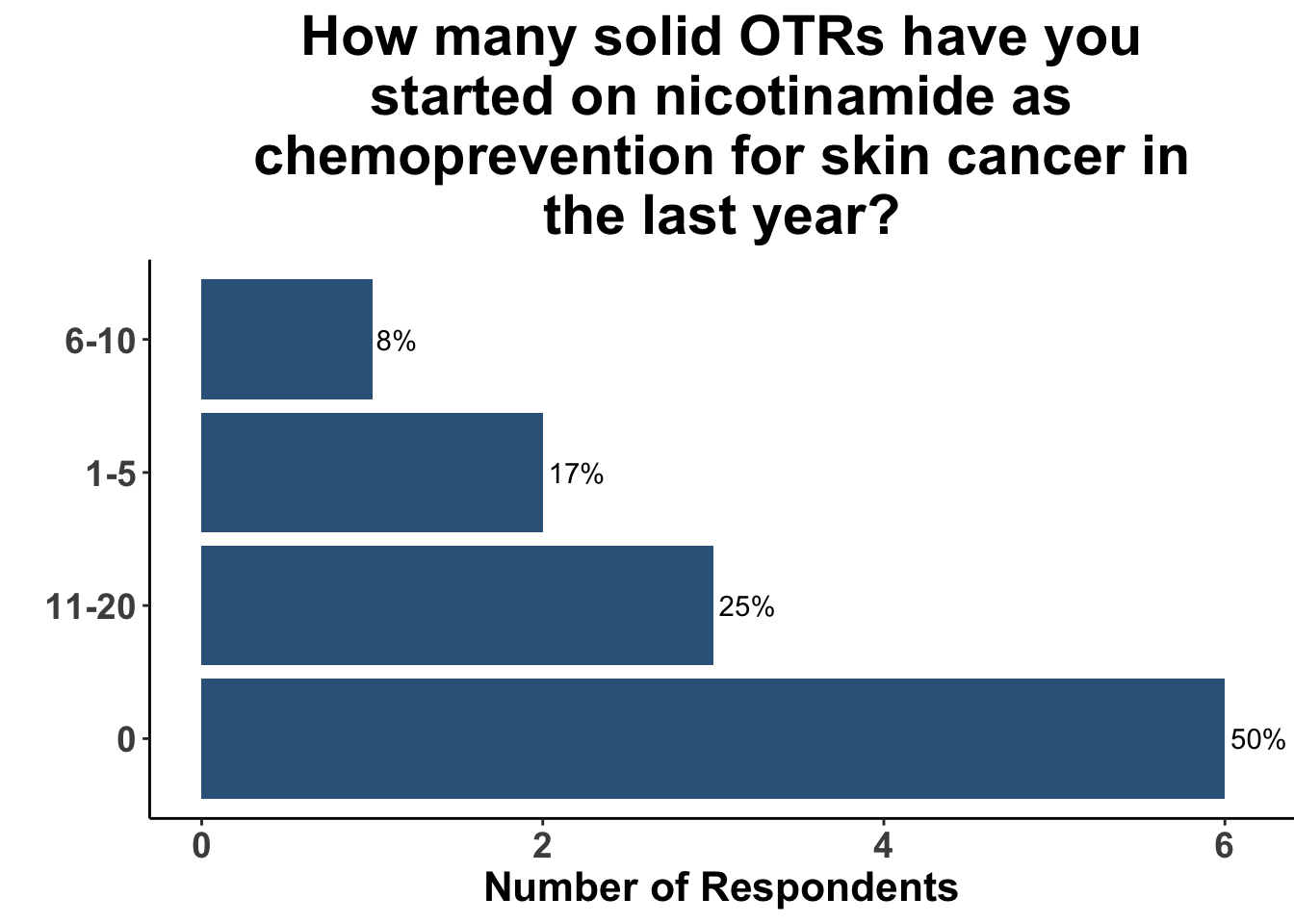
Those participants who would not recommend chemoprevention for the above vignette cited the lack of U.S Food and Drug Administration (FDA) indications for chemoprevention, along with the overall unconvincing body of literature supporting the use of any specific agent, as the most compelling reasons for their decision. Extrapolation from the ONTRAC trial, was posited as rationale for the respondents who selected either nicotinamide monotherapy or dual nicotinamide/acitretin. Indeed, the impact of ONTRAC was exemplified by the fact that the majority (58%) of the clinicians who treat CSCC have used nicotinamide as skin cancer chemoprevention in the last year (Figure 9) and half had used it in SOTRs (Figure 10).
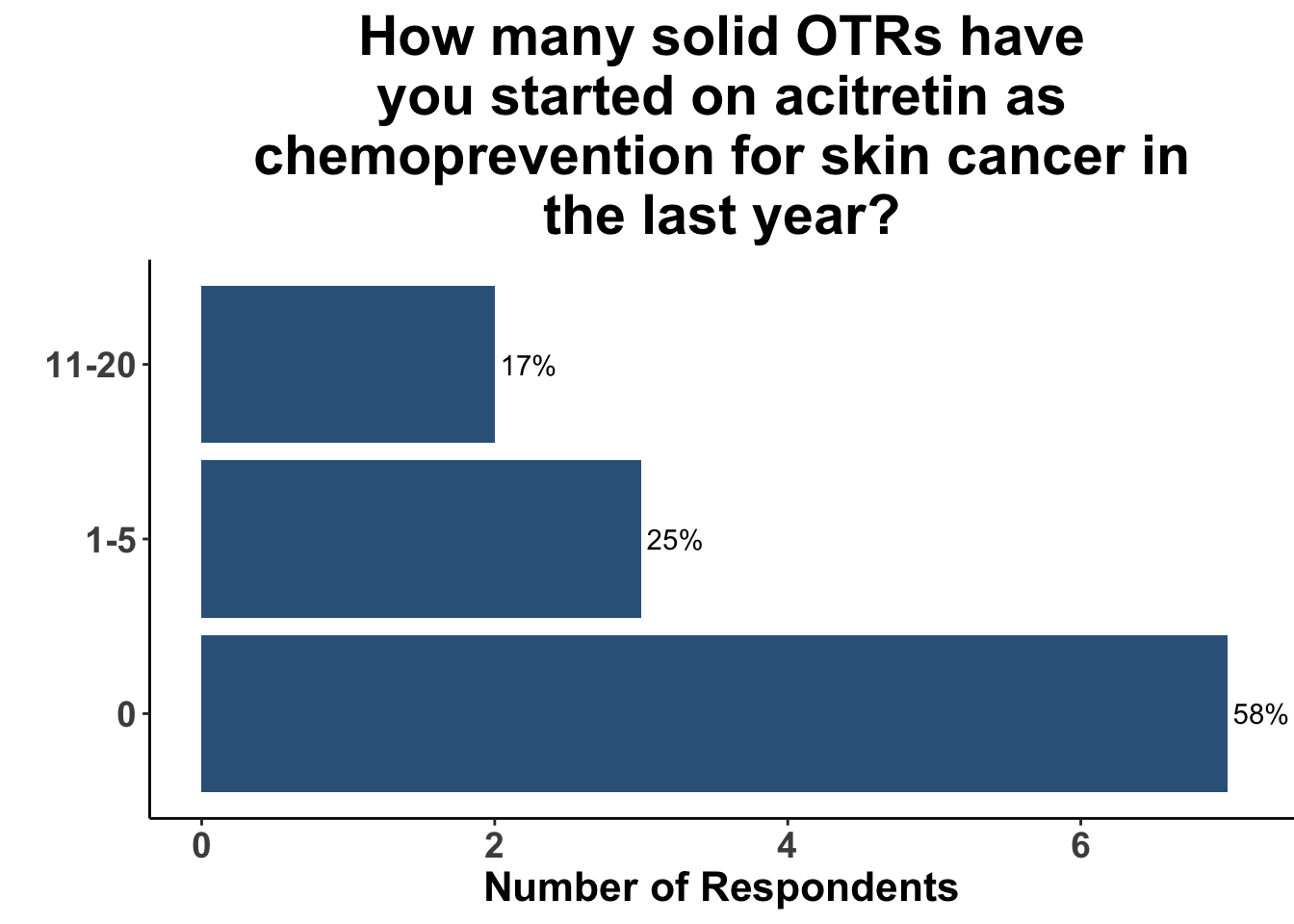
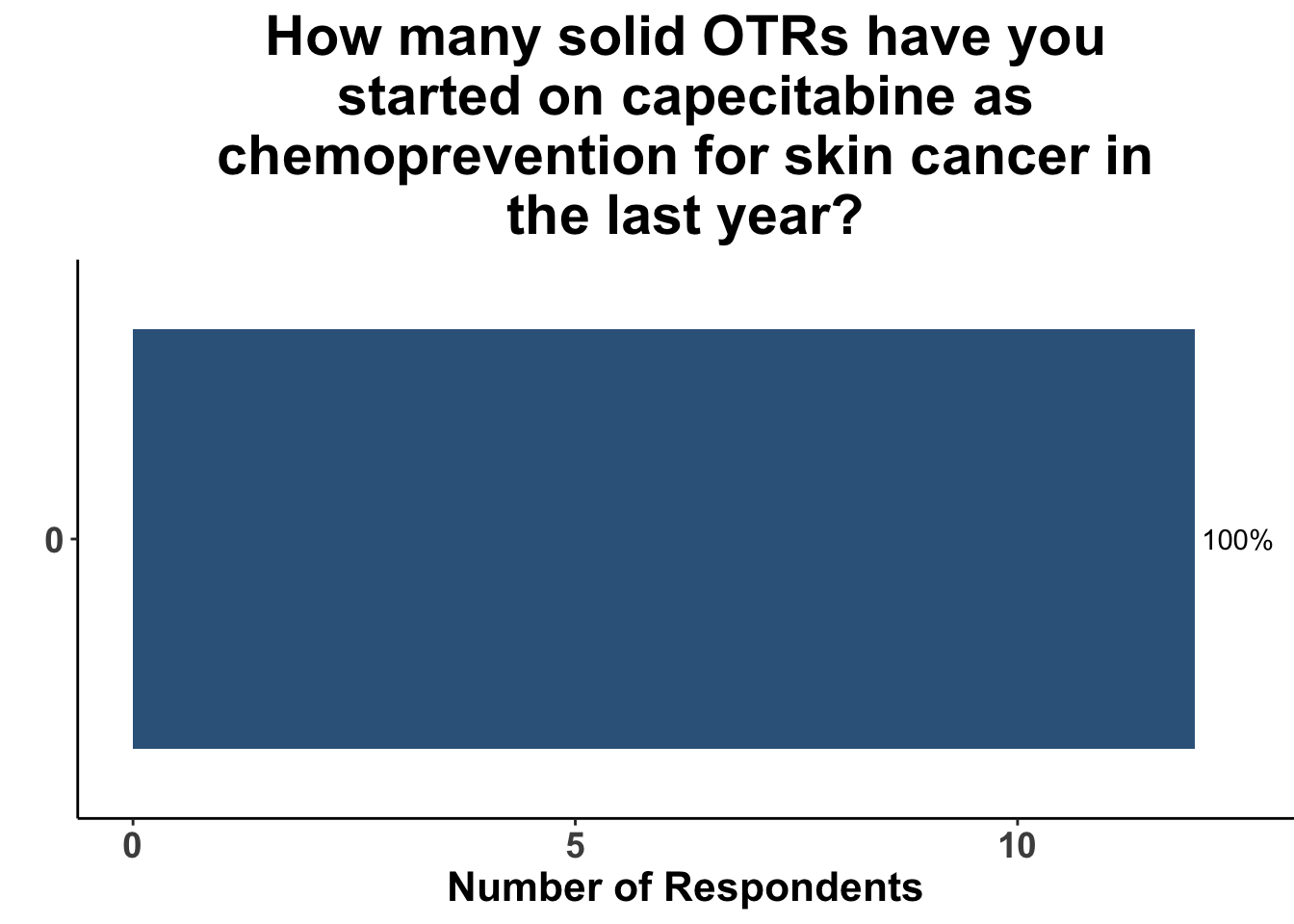
In contrast, the majority of respondents (58%) had not prescribed acitretin as chemoprevention for SOTRs within the last year (Figure 11) and none had recommended capecitabine as a strategy (Figure 12).
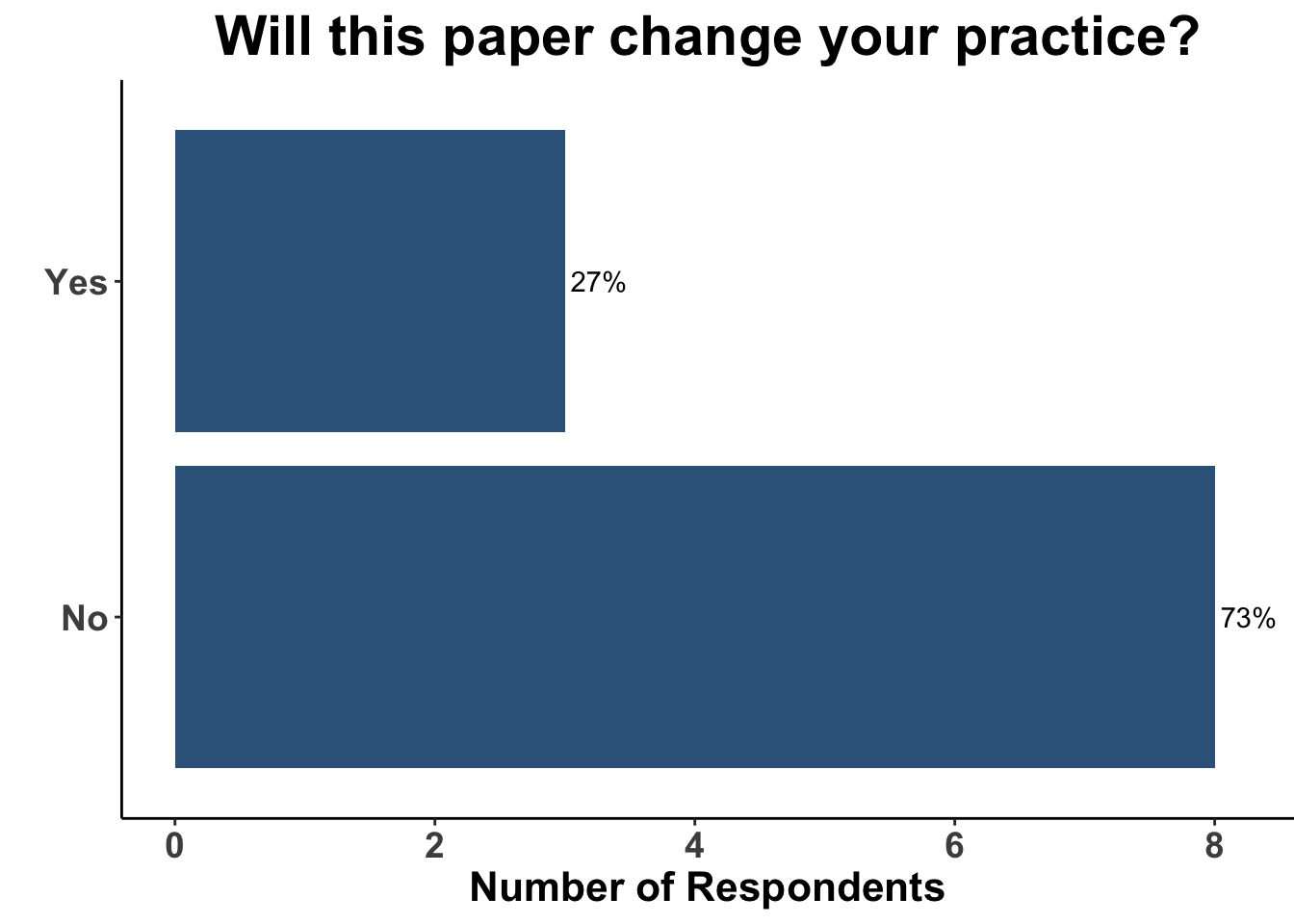
Due to the lack of adoption of nicotinamide as a standard of care chemoprevention strategy, the majority of the clinical attendees (73%) did not believe the data from the ONTRANS trial would change their practice (Figure 13). Only one respondent replied that they would still use nicotinamide in this patient population today (Figure 14), arguing that nicotinamide may still have efficacy in a cohort of SOTRs, especially those who were only recently placed on immunosuppression.
The ONTRANS experience highlights the need to not only reinforce strategies known to be effective for patients at risk for KCs, but it also accentuates the importance of developing novel methods for those at highest risk. Emphasizing lifelong sun protective practices is critical for patients with sun-sensitive phenotypes. Innovative approaches to inducing transplantation tolerance to mitigate the need for chronic immunosuppression for SOTRs are sorely needed. Furthermore, the success of immunotherapy in the treatment keratinocyte carcinomas underscores the role of immune checkpoints, such as Programmed cell death protein 1 (PD-1). Methods to augment anti-tumor immunity in populations that are developing immunosenescence, or are projected to undergo chronic immunosuppression, remain an unmet need. As mentioned previously (David M. Miller and Emerick 2023), immunoprevention (IP) with immune checkpoint inhibitors (ICI), such as anti-PD-1 monoclonal antibodies, have yet to be explored. Given that ICIs can precipitate allograft rejection and loss, IP strategies should initially be investigated in other high-risk populations, such as those with severe field cancerization (also known as actinic neoplasia syndrome) or a history of KCs and hematologic malignancies such as chronic lymphocytic leukemia (CLL). If ICI strategies are deemed safe and effective in these high-risk groups, further investigations in the pre-transplant period could be conducted to evaluate the role of pre-transplantation IP.
Materials and Methods
This Perspectives on the Science piece was published using Quarto®. The survey was conducted using REDCap® (Harris et al. 2009). The figures depicting the survey data were created using R (version 4.0.0) and the tidyverse suite of packages (Wickham et al. 2019), including ggplot2 (Wickham 2016). The flametree image on the “Perspectives on the Science” page was created by the authors (DMM) using the flametree (Navarro 2021) and ggplot2 (Wickham 2016) R packages.
Bibliography
Appendix
Citation
@article{l.kaufman2023,
author = {Howard L. Kaufman and Vishal A. Patel and Sophia Z. Shalhout
and Sameer Gupta and Sonia Cohen and Isaac Brownell and David M.
Miller},
publisher = {Society of Cutaneous Oncology},
title = {Skin {Cancer} {Chemoprevention} for {Transplant} {Recipients}
- {The} {Search} {Continues}},
journal = {Journal of Cutaneous Oncology},
volume = {1},
number = {1},
date = {2023-03-22},
url = {https://journalofcutaneousoncology.io/perspectives/vol_1_issue_1/skin_cancer_chemoprevention_for_transplant_recipients-the_search_continues/},
issn = {pending},
langid = {en}
}