library(skincancerRx)
fda_actions_per_disease_plot(
.xaxistextfont = 13,
.xaxistitlefont = 16,
.yaxistextfont = 14,
.yaxistitlefont = 16,
.plottitlefont = 18,
.plotsubtitlefont = 16,
.geomtextfont = 4)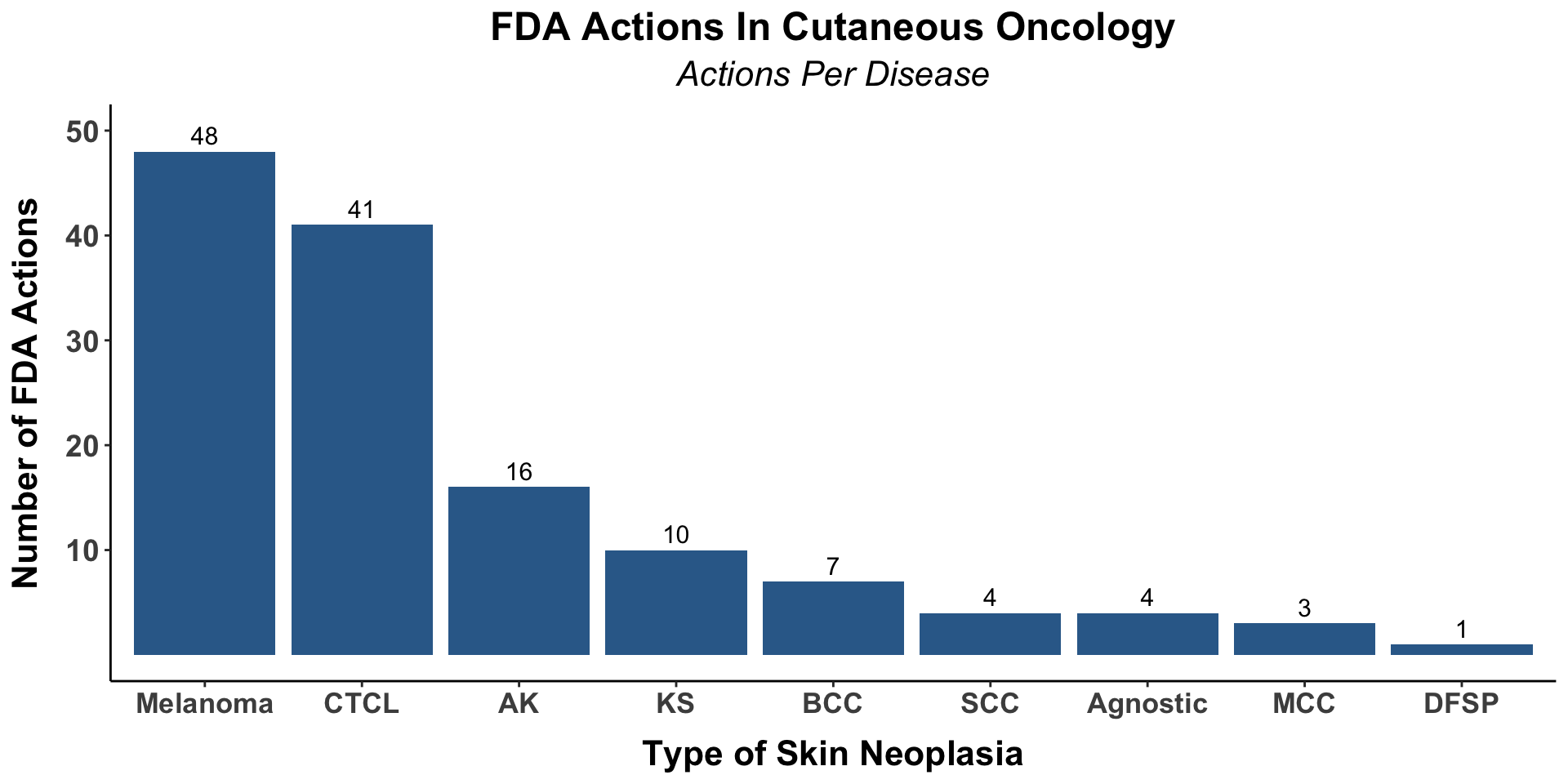
David M. Miller, Sophia Z. Shalhout
December 5, 2022
skincancerRx an R package designed to visualize FDA approvals for skin cancer. The data used in this package was first published as a preprint at themillerlab.io and subsequently accepted at Dermatology Online Journal. In addition, the functions in skincancerRx power the FDA Approvals in Skin Cancer shiny app.
skincancerRx provides a set of verbs that wrangle, process and graph data related to FDA approvals in skin caner:
| Verbs | Function |
|---|---|
| fda_actions_per_disease_plot( ) | generates a bar chart of the number of FDA approvals per type of skin cancer |
| fda_approval_timeliner_df( ) | creates a data frame of FDA approval data that can then be used in the fda_approval_timeliner_plot() function |
| fda_approval_timeliner_plot( ) | creates a data visualization of FDA approvals in skin cancer |
| fda_approval_timeseries_df( ) | creates a data frame of FDA approval data that can then be used in the fda_approval_timeseries_plot() function |
| fda_approval_timeseries_plot( ) | creates a data visualization of FDA approvals in skin cancer |
| response_rate_df( ) | creates a data frame of FDA approval data that can then be used in the response_rate_plot() function |
| response_rate_plot( ) | creates a data visualization of response rates of therapies approved via non-comparator trials |
| table_rx_skin_cancer( ) | creates a table of FDA approvals in skin cancer |
skincancerRx is written in R (version 4.0.0), organized using roxygen2, and utilizes the following packages dplyr, tidyr, readr, stringr, magrittr, plotly, splitstackshape.
To evaluate the evidence used to support labeled claims in skin cancer we reviewed FDA New Drug Application (NDA) or Biological License Application (BLA) reviews, and the US product labels, that are indexed on the FDA website (https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm) . In addition to the product labels and NDA and BLA reviews, data were also obtained from OpenFDA (https://download.open.fda.gov/drug/drugsfda/drug-drugsfda-0001-of-0001.json.zip).
To get a bug fix or to use a feature from the development version, you can install the development version of skincancerRx from GitHub.
devtools::install_github("TheMillerLab/skincancerRx")
library(skincancerRx)
library(kableExtra)
library(knitr)
fda_approval_timeliner_df() |>
kable() %>%
kable_styling(bootstrap_options = c("striped","hover"),
fixed_thead = T) %>%
scroll_box(height = "400px",
width = "100%")| date | name | Dz | Indication_brief | Indication_short | Mechanism | Mechanism_long | Mechanistic_Class | y | hover.a | hover.b | hover.c | hover.d | hover |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1949-03-15 | Mechlorethamine Hydrochloride | CTCL | Mycosis Fungoides | Other | Alkylating Agent | Alkylating Agent | Cytotoxic Agent | 1 | <b>Drug:</b> Mechlorethamine Hydrochloride | <b>Date of Approval:</b> 1949-03-15 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Alkylating Agent | <b>Drug:</b> Mechlorethamine Hydrochloride<br><b>Date of Approval:</b> 1949-03-15<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Alkylating Agent |
| 1950-06-13 | Cortisone Acetate | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 2 | <b>Drug:</b> Cortisone Acetate | <b>Date of Approval:</b> 1950-06-13 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Cortisone Acetate<br><b>Date of Approval:</b> 1950-06-13<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1950-12-04 | Cortisone Acetate | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 3 | <b>Drug:</b> Cortisone Acetate | <b>Date of Approval:</b> 1950-12-04 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Cortisone Acetate<br><b>Date of Approval:</b> 1950-12-04<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1952-12-15 | Hydrocortisone | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 4 | <b>Drug:</b> Hydrocortisone | <b>Date of Approval:</b> 1952-12-15 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Hydrocortisone<br><b>Date of Approval:</b> 1952-12-15<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1953-12-07 | Methotrexate Sodium | CTCL | Mycosis Fungoides | Other | Antimetabolite | Antimetabolite | Cytotoxic Agent | 5 | <b>Drug:</b> Methotrexate Sodium | <b>Date of Approval:</b> 1953-12-07 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Antimetabolite | <b>Drug:</b> Methotrexate Sodium<br><b>Date of Approval:</b> 1953-12-07<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Antimetabolite |
| 1955-02-21 | Prednisone | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 6 | <b>Drug:</b> Prednisone | <b>Date of Approval:</b> 1955-02-21 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Prednisone<br><b>Date of Approval:</b> 1955-02-21<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1955-04-27 | Hydrocortisone Sodium Succinate | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 7 | <b>Drug:</b> Hydrocortisone Sodium Succinate | <b>Date of Approval:</b> 1955-04-27 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Hydrocortisone Sodium Succinate<br><b>Date of Approval:</b> 1955-04-27<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1955-06-21 | Prednisolone | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 8 | <b>Drug:</b> Prednisolone | <b>Date of Approval:</b> 1955-06-21 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Prednisolone<br><b>Date of Approval:</b> 1955-06-21<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1957-10-24 | Methylprednisolone | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 9 | <b>Drug:</b> Methylprednisolone | <b>Date of Approval:</b> 1957-10-24 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Methylprednisolone<br><b>Date of Approval:</b> 1957-10-24<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1957-12-03 | Triamcinolone | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 10 | <b>Drug:</b> Triamcinolone | <b>Date of Approval:</b> 1957-12-03 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Triamcinolone<br><b>Date of Approval:</b> 1957-12-03<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1958-10-30 | Dexamethasone | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 11 | <b>Drug:</b> Dexamethasone | <b>Date of Approval:</b> 1958-10-30 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Dexamethasone<br><b>Date of Approval:</b> 1958-10-30<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1959-03-12 | Triamcinolone Diacetate | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 12 | <b>Drug:</b> Triamcinolone Diacetate | <b>Date of Approval:</b> 1959-03-12 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Triamcinolone Diacetate<br><b>Date of Approval:</b> 1959-03-12<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1959-05-18 | Methylprednisolone Sodium Succinate | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 13 | <b>Drug:</b> Methylprednisolone Sodium Succinate | <b>Date of Approval:</b> 1959-05-18 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Methylprednisolone Sodium Succinate<br><b>Date of Approval:</b> 1959-05-18<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1959-05-27 | Methylprednisolone Acetate | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 14 | <b>Drug:</b> Methylprednisolone Acetate | <b>Date of Approval:</b> 1959-05-27 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Methylprednisolone Acetate<br><b>Date of Approval:</b> 1959-05-27<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1959-08-10 | Methotrexate Sodium | CTCL | Mycosis Fungoides | Other | Antimetabolite | Antimetabolite | Cytotoxic Agent | 15 | <b>Drug:</b> Methotrexate Sodium | <b>Date of Approval:</b> 1959-08-10 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Antimetabolite | <b>Drug:</b> Methotrexate Sodium<br><b>Date of Approval:</b> 1959-08-10<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Antimetabolite |
| 1959-10-06 | Dexamethasone Sodium Phosphate | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 16 | <b>Drug:</b> Dexamethasone Sodium Phosphate | <b>Date of Approval:</b> 1959-10-06 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Dexamethasone Sodium Phosphate<br><b>Date of Approval:</b> 1959-10-06<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1959-11-16 | Cyclophosphamide | CTCL | Mycosis Fungoides | Other | Alkylating Agent | Alkylating Agent | Cytotoxic Agent | 17 | <b>Drug:</b> Cyclophosphamide | <b>Date of Approval:</b> 1959-11-16 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Alkylating Agent | <b>Drug:</b> Cyclophosphamide<br><b>Date of Approval:</b> 1959-11-16<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Alkylating Agent |
| 1959-11-16 | Cyclophosphamide | CTCL | Mycosis Fungoides | Other | Alkylating Agent | Alkylating Agent | Cytotoxic Agent | 18 | <b>Drug:</b> Cyclophosphamide | <b>Date of Approval:</b> 1959-11-16 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Alkylating Agent | <b>Drug:</b> Cyclophosphamide<br><b>Date of Approval:</b> 1959-11-16<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Alkylating Agent |
| 1960-06-21 | Methylprednisolone Acetate | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 19 | <b>Drug:</b> Methylprednisolone Acetate | <b>Date of Approval:</b> 1960-06-21 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Methylprednisolone Acetate<br><b>Date of Approval:</b> 1960-06-21<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1960-07-07 | Dexamethasone | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 20 | <b>Drug:</b> Dexamethasone | <b>Date of Approval:</b> 1960-07-07 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Dexamethasone<br><b>Date of Approval:</b> 1960-07-07<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1961-04-17 | Betamethasone | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 21 | <b>Drug:</b> Betamethasone | <b>Date of Approval:</b> 1961-04-17 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Betamethasone<br><b>Date of Approval:</b> 1961-04-17<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1961-09-05 | Triamcinolone Diacetate | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 22 | <b>Drug:</b> Triamcinolone Diacetate | <b>Date of Approval:</b> 1961-09-05 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Triamcinolone Diacetate<br><b>Date of Approval:</b> 1961-09-05<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1965-02-01 | Triamcinolone Acetonide | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 23 | <b>Drug:</b> Triamcinolone Acetonide | <b>Date of Approval:</b> 1965-02-01 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Triamcinolone Acetonide<br><b>Date of Approval:</b> 1965-02-01<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1965-03-03 | Betamethasone Acetate | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 24 | <b>Drug:</b> Betamethasone Acetate | <b>Date of Approval:</b> 1965-03-03 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Betamethasone Acetate<br><b>Date of Approval:</b> 1965-03-03<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1965-11-25 | Vinblastine Sulfate | CTCL | Mycosis Fungoides | Other | Microtubule Inhibitor | Microtubule Inhibitor | Cytotoxic Agent | 25 | <b>Drug:</b> Vinblastine Sulfate | <b>Date of Approval:</b> 1965-11-25 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Microtubule Inhibitor | <b>Drug:</b> Vinblastine Sulfate<br><b>Date of Approval:</b> 1965-11-25<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Microtubule Inhibitor |
| 1965-11-25 | Vinblastine Sulfate | KS | Kaposi's Sarcoma | Other | Microtubule Inhibitor | Microtubule Inhibitor | Cytotoxic Agent | 26 | <b>Drug:</b> Vinblastine Sulfate | <b>Date of Approval:</b> 1965-11-25 | <b>Indication:</b> Kaposi's Sarcoma | <b>Mechanism:</b> Microtubule Inhibitor | <b>Drug:</b> Vinblastine Sulfate<br><b>Date of Approval:</b> 1965-11-25<br><b>Indication:</b> Kaposi's Sarcoma<br><b>Mechanism:</b> Microtubule Inhibitor |
| 1967-12-07 | Hydroxyurea | Melanoma | Melanoma | Other | Antimetabolite | Antimetabolite | Cytotoxic Agent | 27 | <b>Drug:</b> Hydroxyurea | <b>Date of Approval:</b> 1967-12-07 | <b>Indication:</b> Melanoma | <b>Mechanism:</b> Antimetabolite | <b>Drug:</b> Hydroxyurea<br><b>Date of Approval:</b> 1967-12-07<br><b>Indication:</b> Melanoma<br><b>Mechanism:</b> Antimetabolite |
| 1970-07-29 | Fluorouracil | AK | Multiple Actinic Keratoses | Other | Antimetabolite | Antimetabolite | Cytotoxic Agent | 28 | <b>Drug:</b> Fluorouracil | <b>Date of Approval:</b> 1970-07-29 | <b>Indication:</b> Multiple Actinic Keratoses | <b>Mechanism:</b> Antimetabolite | <b>Drug:</b> Fluorouracil<br><b>Date of Approval:</b> 1970-07-29<br><b>Indication:</b> Multiple Actinic Keratoses<br><b>Mechanism:</b> Antimetabolite |
| 1971-08-06 | Fluorouracil | AK | Actinic Keratoses | Other | Antimetabolite | Antimetabolite | Cytotoxic Agent | 29 | <b>Drug:</b> Fluorouracil | <b>Date of Approval:</b> 1971-08-06 | <b>Indication:</b> Actinic Keratoses | <b>Mechanism:</b> Antimetabolite | <b>Drug:</b> Fluorouracil<br><b>Date of Approval:</b> 1971-08-06<br><b>Indication:</b> Actinic Keratoses<br><b>Mechanism:</b> Antimetabolite |
| 1975-05-27 | Dacarbazine | Melanoma | Metastatic Malignant Melanoma | Metastatic | Alkylating Agent | Alkylating Agent | Cytotoxic Agent | 30 | <b>Drug:</b> Dacarbazine | <b>Date of Approval:</b> 1975-05-27 | <b>Indication:</b> Metastatic Malignant Melanoma | <b>Mechanism:</b> Alkylating Agent | <b>Drug:</b> Dacarbazine<br><b>Date of Approval:</b> 1975-05-27<br><b>Indication:</b> Metastatic Malignant Melanoma<br><b>Mechanism:</b> Alkylating Agent |
| 1975-06-30 | Fluorouracil | BCC | Superficial Basal Cell Carcinoma | Other | Antimetabolite | Antimetabolite | Cytotoxic Agent | 31 | <b>Drug:</b> Fluorouracil | <b>Date of Approval:</b> 1975-06-30 | <b>Indication:</b> Superficial Basal Cell Carcinoma | <b>Mechanism:</b> Antimetabolite | <b>Drug:</b> Fluorouracil<br><b>Date of Approval:</b> 1975-06-30<br><b>Indication:</b> Superficial Basal Cell Carcinoma<br><b>Mechanism:</b> Antimetabolite |
| 1986-05-28 | Prednisolone Sodium Phosphate | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 32 | <b>Drug:</b> Prednisolone Sodium Phosphate | <b>Date of Approval:</b> 1986-05-28 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Prednisolone Sodium Phosphate<br><b>Date of Approval:</b> 1986-05-28<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 1988-03-23 | Methoxsalen | CTCL | Refractory Cutaneous T-Cell Lymphoma | Refractory | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 33 | <b>Drug:</b> Methoxsalen | <b>Date of Approval:</b> 1988-03-23 | <b>Indication:</b> Refractory Cutaneous T-Cell Lymphoma | <b>Mechanism:</b> Phototoxic | <b>Drug:</b> Methoxsalen<br><b>Date of Approval:</b> 1988-03-23<br><b>Indication:</b> Refractory Cutaneous T-Cell Lymphoma<br><b>Mechanism:</b> Phototoxic |
| 1988-11-21 | Interferon Alfa-2b | KS | AIDS-Related Kaposi's Sarcoma | Other | Cytokine | Cytokine | Immunotherapy | 34 | <b>Drug:</b> Interferon Alfa-2b | <b>Date of Approval:</b> 1988-11-21 | <b>Indication:</b> AIDS-Related Kaposi's Sarcoma | <b>Mechanism:</b> Cytokine | <b>Drug:</b> Interferon Alfa-2b<br><b>Date of Approval:</b> 1988-11-21<br><b>Indication:</b> AIDS-Related Kaposi's Sarcoma<br><b>Mechanism:</b> Cytokine |
| 1988-11-21 | Interferon Alfa-2a | KS | AIDS-Related Kaposi's Sarcoma | Other | Cytokine | Cytokine | Immunotherapy | 35 | <b>Drug:</b> Interferon Alfa-2a | <b>Date of Approval:</b> 1988-11-21 | <b>Indication:</b> AIDS-Related Kaposi's Sarcoma | <b>Mechanism:</b> Cytokine | <b>Drug:</b> Interferon Alfa-2a<br><b>Date of Approval:</b> 1988-11-21<br><b>Indication:</b> AIDS-Related Kaposi's Sarcoma<br><b>Mechanism:</b> Cytokine |
| 1995-11-17 | Doxorubicin Hydrochloride | KS | Accelerated Approval for Refractory AIDS-Related Kaposi's Sarcoma | Refractory | Topoisomerase Inhibitor | Topoisomerase Inhibitor | Cytotoxic Agent | 36 | <b>Drug:</b> Doxorubicin Hydrochloride | <b>Date of Approval:</b> 1995-11-17 | <b>Indication:</b> Accelerated Approval for Refractory AIDS-Related Kaposi's Sarcoma | <b>Mechanism:</b> Topoisomerase Inhibitor | <b>Drug:</b> Doxorubicin Hydrochloride<br><b>Date of Approval:</b> 1995-11-17<br><b>Indication:</b> Accelerated Approval for Refractory AIDS-Related Kaposi's Sarcoma<br><b>Mechanism:</b> Topoisomerase Inhibitor |
| 1995-12-05 | Interferon Alfa-2b | Melanoma | Adjuvant Melanoma | Adjuvant | Cytokine | Cytokine | Immunotherapy | 37 | <b>Drug:</b> Interferon Alfa-2b | <b>Date of Approval:</b> 1995-12-05 | <b>Indication:</b> Adjuvant Melanoma | <b>Mechanism:</b> Cytokine | <b>Drug:</b> Interferon Alfa-2b<br><b>Date of Approval:</b> 1995-12-05<br><b>Indication:</b> Adjuvant Melanoma<br><b>Mechanism:</b> Cytokine |
| 1996-04-08 | Daunorubicin Citrate | KS | HIV-associated Kaposi's Sarcoma - First Line | Other | Topoisomerase Inhibitor | Topoisomerase Inhibitor | Cytotoxic Agent | 38 | <b>Drug:</b> Daunorubicin Citrate | <b>Date of Approval:</b> 1996-04-08 | <b>Indication:</b> HIV-associated Kaposi's Sarcoma - First Line | <b>Mechanism:</b> Topoisomerase Inhibitor | <b>Drug:</b> Daunorubicin Citrate<br><b>Date of Approval:</b> 1996-04-08<br><b>Indication:</b> HIV-associated Kaposi's Sarcoma - First Line<br><b>Mechanism:</b> Topoisomerase Inhibitor |
| 1997-08-04 | Paclitaxel | KS | AIDS-Related Kaposi's Sarcoma - Second Line | Refractory | Microtubule Stabilizer | Microtubule Stabilizer | Cytotoxic Agent | 39 | <b>Drug:</b> Paclitaxel | <b>Date of Approval:</b> 1997-08-04 | <b>Indication:</b> AIDS-Related Kaposi's Sarcoma - Second Line | <b>Mechanism:</b> Microtubule Stabilizer | <b>Drug:</b> Paclitaxel<br><b>Date of Approval:</b> 1997-08-04<br><b>Indication:</b> AIDS-Related Kaposi's Sarcoma - Second Line<br><b>Mechanism:</b> Microtubule Stabilizer |
| 1998-01-09 | Aldesleukin | Melanoma | Metastatic Melanoma | Metastatic | Cytokine | Cytokine | Immunotherapy | 40 | <b>Drug:</b> Aldesleukin | <b>Date of Approval:</b> 1998-01-09 | <b>Indication:</b> Metastatic Melanoma | <b>Mechanism:</b> Cytokine | <b>Drug:</b> Aldesleukin<br><b>Date of Approval:</b> 1998-01-09<br><b>Indication:</b> Metastatic Melanoma<br><b>Mechanism:</b> Cytokine |
| 1998-12-17 | Prednisolone Sodium Phosphate | CTCL | Expansion to include pediatric populations | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 41 | <b>Drug:</b> Prednisolone Sodium Phosphate | <b>Date of Approval:</b> 1998-12-17 | <b>Indication:</b> Expansion to include pediatric populations | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Prednisolone Sodium Phosphate<br><b>Date of Approval:</b> 1998-12-17<br><b>Indication:</b> Expansion to include pediatric populations<br><b>Mechanism:</b> Glucocorticoid |
| 1999-02-02 | Alitretinoin | KS | AIDS-Related Kaposi's Sarcoma - Cutaneous Lesions Only | Other | Retinoid | Retinoid | Retinoid | 42 | <b>Drug:</b> Alitretinoin | <b>Date of Approval:</b> 1999-02-02 | <b>Indication:</b> AIDS-Related Kaposi's Sarcoma - Cutaneous Lesions Only | <b>Mechanism:</b> Retinoid | <b>Drug:</b> Alitretinoin<br><b>Date of Approval:</b> 1999-02-02<br><b>Indication:</b> AIDS-Related Kaposi's Sarcoma - Cutaneous Lesions Only<br><b>Mechanism:</b> Retinoid |
| 1999-02-05 | Denileukin Diftitox | CTCL | Accelerated Approval for Persistent or Recurrent CD25+ Cutaneous T-Cell Lymphoma | Other | Cytokine-Cytotoxin | Cytokine-Cytotoxin | Cytotoxic Agent | 43 | <b>Drug:</b> Denileukin Diftitox | <b>Date of Approval:</b> 1999-02-05 | <b>Indication:</b> Accelerated Approval for Persistent or Recurrent CD25+ Cutaneous T-Cell Lymphoma | <b>Mechanism:</b> Cytokine-Cytotoxin | <b>Drug:</b> Denileukin Diftitox<br><b>Date of Approval:</b> 1999-02-05<br><b>Indication:</b> Accelerated Approval for Persistent or Recurrent CD25+ Cutaneous T-Cell Lymphoma<br><b>Mechanism:</b> Cytokine-Cytotoxin |
| 1999-02-25 | Methoxsalen | CTCL | Refractory Cutaneous T-Cell Lymphoma | Refractory | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 44 | <b>Drug:</b> Methoxsalen | <b>Date of Approval:</b> 1999-02-25 | <b>Indication:</b> Refractory Cutaneous T-Cell Lymphoma | <b>Mechanism:</b> Phototoxic | <b>Drug:</b> Methoxsalen<br><b>Date of Approval:</b> 1999-02-25<br><b>Indication:</b> Refractory Cutaneous T-Cell Lymphoma<br><b>Mechanism:</b> Phototoxic |
| 1999-12-03 | Aminolevulinic Acid Hydrochloride | AK | Non-Hyperkeratotic Actinic Keratoses of the Face or Scalp | Other | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 45 | <b>Drug:</b> Aminolevulinic Acid Hydrochloride | <b>Date of Approval:</b> 1999-12-03 | <b>Indication:</b> Non-Hyperkeratotic Actinic Keratoses of the Face or Scalp | <b>Mechanism:</b> Phototoxic | <b>Drug:</b> Aminolevulinic Acid Hydrochloride<br><b>Date of Approval:</b> 1999-12-03<br><b>Indication:</b> Non-Hyperkeratotic Actinic Keratoses of the Face or Scalp<br><b>Mechanism:</b> Phototoxic |
| 1999-12-29 | Bexarotene | CTCL | Refractory Cutaneous T-Cell Lymphoma | Refractory | Retinoid | Retinoid | Retinoid | 46 | <b>Drug:</b> Bexarotene | <b>Date of Approval:</b> 1999-12-29 | <b>Indication:</b> Refractory Cutaneous T-Cell Lymphoma | <b>Mechanism:</b> Retinoid | <b>Drug:</b> Bexarotene<br><b>Date of Approval:</b> 1999-12-29<br><b>Indication:</b> Refractory Cutaneous T-Cell Lymphoma<br><b>Mechanism:</b> Retinoid |
| 2000-06-28 | Bexarotene | CTCL | Refractory Stage IA and IB Cutaneous T-Cell Lymphoma | Refractory | Retinoid | Retinoid | Retinoid | 47 | <b>Drug:</b> Bexarotene | <b>Date of Approval:</b> 2000-06-28 | <b>Indication:</b> Refractory Stage IA and IB Cutaneous T-Cell Lymphoma | <b>Mechanism:</b> Retinoid | <b>Drug:</b> Bexarotene<br><b>Date of Approval:</b> 2000-06-28<br><b>Indication:</b> Refractory Stage IA and IB Cutaneous T-Cell Lymphoma<br><b>Mechanism:</b> Retinoid |
| 2000-10-16 | Diclofenac Sodium | AK | Actinic Keratoses | Other | NSAID | NSAID | NSAID | 48 | <b>Drug:</b> Diclofenac Sodium | <b>Date of Approval:</b> 2000-10-16 | <b>Indication:</b> Actinic Keratoses | <b>Mechanism:</b> NSAID | <b>Drug:</b> Diclofenac Sodium<br><b>Date of Approval:</b> 2000-10-16<br><b>Indication:</b> Actinic Keratoses<br><b>Mechanism:</b> NSAID |
| 2000-10-27 | Fluorouracil | AK | Multiple Actinic Keratoses of the Face and Anterior Scalp | Other | Antimetabolite | Antimetabolite | Cytotoxic Agent | 49 | <b>Drug:</b> Fluorouracil | <b>Date of Approval:</b> 2000-10-27 | <b>Indication:</b> Multiple Actinic Keratoses of the Face and Anterior Scalp | <b>Mechanism:</b> Antimetabolite | <b>Drug:</b> Fluorouracil<br><b>Date of Approval:</b> 2000-10-27<br><b>Indication:</b> Multiple Actinic Keratoses of the Face and Anterior Scalp<br><b>Mechanism:</b> Antimetabolite |
| 2004-03-02 | Imiquimod | AK | Nonhypertrophic Actinic Keratoses on the Face or Scalp | Other | TLR Agonist | TLR Agonist | Immunotherapy | 50 | <b>Drug:</b> Imiquimod | <b>Date of Approval:</b> 2004-03-02 | <b>Indication:</b> Nonhypertrophic Actinic Keratoses on the Face or Scalp | <b>Mechanism:</b> TLR Agonist | <b>Drug:</b> Imiquimod<br><b>Date of Approval:</b> 2004-03-02<br><b>Indication:</b> Nonhypertrophic Actinic Keratoses on the Face or Scalp<br><b>Mechanism:</b> TLR Agonist |
| 2004-07-14 | Imiquimod | BCC | Superficial Basal Cell Carcinoma | Other | TLR Agonist | TLR Agonist | Immunotherapy | 51 | <b>Drug:</b> Imiquimod | <b>Date of Approval:</b> 2004-07-14 | <b>Indication:</b> Superficial Basal Cell Carcinoma | <b>Mechanism:</b> TLR Agonist | <b>Drug:</b> Imiquimod<br><b>Date of Approval:</b> 2004-07-14<br><b>Indication:</b> Superficial Basal Cell Carcinoma<br><b>Mechanism:</b> TLR Agonist |
| 2004-07-27 | Methyl Aminolevulinate Hydrochloride | AK | Non-Keratotic Actinic Keratoses of the Face and Scalp | Other | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 52 | <b>Drug:</b> Methyl Aminolevulinate Hydrochloride | <b>Date of Approval:</b> 2004-07-27 | <b>Indication:</b> Non-Keratotic Actinic Keratoses of the Face and Scalp | <b>Mechanism:</b> Phototoxic | <b>Drug:</b> Methyl Aminolevulinate Hydrochloride<br><b>Date of Approval:</b> 2004-07-27<br><b>Indication:</b> Non-Keratotic Actinic Keratoses of the Face and Scalp<br><b>Mechanism:</b> Phototoxic |
| 2006-06-01 | Prednisolone Sodium Phosphate | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 53 | <b>Drug:</b> Prednisolone Sodium Phosphate | <b>Date of Approval:</b> 2006-06-01 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Prednisolone Sodium Phosphate<br><b>Date of Approval:</b> 2006-06-01<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 2006-10-06 | Vorinostat | CTCL | Refractory Cutaneous T-Cell Lymphoma | Refractory | HDAC Inhibitor | HDAC Inhibitor | Targeted Therapy | 54 | <b>Drug:</b> Vorinostat | <b>Date of Approval:</b> 2006-10-06 | <b>Indication:</b> Refractory Cutaneous T-Cell Lymphoma | <b>Mechanism:</b> HDAC Inhibitor | <b>Drug:</b> Vorinostat<br><b>Date of Approval:</b> 2006-10-06<br><b>Indication:</b> Refractory Cutaneous T-Cell Lymphoma<br><b>Mechanism:</b> HDAC Inhibitor |
| 2006-10-19 | Imatinib Mesylate | DFSP | Unresectable, Recurrent and/or Metastatic Dermatofibrosarcoma Protuberans | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 55 | <b>Drug:</b> Imatinib Mesylate | <b>Date of Approval:</b> 2006-10-19 | <b>Indication:</b> Unresectable, Recurrent and/or Metastatic Dermatofibrosarcoma Protuberans | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Imatinib Mesylate<br><b>Date of Approval:</b> 2006-10-19<br><b>Indication:</b> Unresectable, Recurrent and/or Metastatic Dermatofibrosarcoma Protuberans<br><b>Mechanism:</b> Kinase Inhibitor |
| 2008-01-17 | Prednisolone Acetate | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 56 | <b>Drug:</b> Prednisolone Acetate | <b>Date of Approval:</b> 2008-01-17 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Prednisolone Acetate<br><b>Date of Approval:</b> 2008-01-17<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 2008-06-10 | Doxorubicin Hydrochloride | KS | Regular Aprroval for Refractory AIDS-Related Kaposi's Sarcoma | Refractory | Topoisomerase Inhibitor | Topoisomerase Inhibitor | Cytotoxic Agent | 57 | <b>Drug:</b> Doxorubicin Hydrochloride | <b>Date of Approval:</b> 2008-06-10 | <b>Indication:</b> Regular Aprroval for Refractory AIDS-Related Kaposi's Sarcoma | <b>Mechanism:</b> Topoisomerase Inhibitor | <b>Drug:</b> Doxorubicin Hydrochloride<br><b>Date of Approval:</b> 2008-06-10<br><b>Indication:</b> Regular Aprroval for Refractory AIDS-Related Kaposi's Sarcoma<br><b>Mechanism:</b> Topoisomerase Inhibitor |
| 2008-06-16 | Triamcinolone Acetonide | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 58 | <b>Drug:</b> Triamcinolone Acetonide | <b>Date of Approval:</b> 2008-06-16 | <b>Indication:</b> Mycosis Fungoides | <b>Mechanism:</b> Glucocorticoid | <b>Drug:</b> Triamcinolone Acetonide<br><b>Date of Approval:</b> 2008-06-16<br><b>Indication:</b> Mycosis Fungoides<br><b>Mechanism:</b> Glucocorticoid |
| 2008-06-26 | Methyl Aminolevulinate Hydrochloride | AK | Use of Metvixia with a new Lamp (Aktilite) in AKs | Other | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 59 | <b>Drug:</b> Methyl Aminolevulinate Hydrochloride | <b>Date of Approval:</b> 2008-06-26 | <b>Indication:</b> Use of Metvixia with a new Lamp (Aktilite) in AKs | <b>Mechanism:</b> Phototoxic | <b>Drug:</b> Methyl Aminolevulinate Hydrochloride<br><b>Date of Approval:</b> 2008-06-26<br><b>Indication:</b> Use of Metvixia with a new Lamp (Aktilite) in AKs<br><b>Mechanism:</b> Phototoxic |
| 2008-10-15 | Denileukin Diftitox | CTCL | Regular Approval for Persistent or Recurrent CD25+ Cutaneous T-Cell Lymphoma | Other | Cytokine-Cytotoxin | Cytokine-Cytotoxin | Cytotoxic Agent | 60 | <b>Drug:</b> Denileukin Diftitox | <b>Date of Approval:</b> 2008-10-15 | <b>Indication:</b> Regular Approval for Persistent or Recurrent CD25+ Cutaneous T-Cell Lymphoma | <b>Mechanism:</b> Cytokine-Cytotoxin | <b>Drug:</b> Denileukin Diftitox<br><b>Date of Approval:</b> 2008-10-15<br><b>Indication:</b> Regular Approval for Persistent or Recurrent CD25+ Cutaneous T-Cell Lymphoma<br><b>Mechanism:</b> Cytokine-Cytotoxin |
| 2009-11-05 | Romidepsin | CTCL | Refractory Cutaneous T-Cell Lymphoma | Refractory | HDAC Inhibitor | HDAC Inhibitor | HDAC Inhibitor | 61 | <b>Drug:</b> Romidepsin | <b>Date of Approval:</b> 2009-11-05 | <b>Indication:</b> Refractory Cutaneous T-Cell Lymphoma | <b>Mechanism:</b> HDAC Inhibitor | <b>Drug:</b> Romidepsin<br><b>Date of Approval:</b> 2009-11-05<br><b>Indication:</b> Refractory Cutaneous T-Cell Lymphoma<br><b>Mechanism:</b> HDAC Inhibitor |
| 2010-03-12 | Aminolevulinic Acid Hydrochloride | AK | Allow user to mix contents for 30 seconds prior to use & to use 'Kerastick Krusher' | Other | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 62 | <b>Drug:</b> Aminolevulinic Acid Hydrochloride | <b>Date of Approval:</b> 2010-03-12 | <b>Indication:</b> Allow user to mix contents for 30 seconds prior to use & to use 'Kerastick Krusher' | <b>Mechanism:</b> Phototoxic | <b>Drug:</b> Aminolevulinic Acid Hydrochloride<br><b>Date of Approval:</b> 2010-03-12<br><b>Indication:</b> Allow user to mix contents for 30 seconds prior to use & to use 'Kerastick Krusher'<br><b>Mechanism:</b> Phototoxic |
| 2010-03-25 | Imiquimod | AK | Use of 3.75% form for Actinic Keratoses of the Full Face or Balding Scalp | Other | TLR Agonist | TLR Agonist | Immunotherapy | 63 | <b>Drug:</b> Imiquimod | <b>Date of Approval:</b> 2010-03-25 | <b>Indication:</b> Use of 3.75% form for Actinic Keratoses of the Full Face or Balding Scalp | <b>Mechanism:</b> TLR Agonist | <b>Drug:</b> Imiquimod<br><b>Date of Approval:</b> 2010-03-25<br><b>Indication:</b> Use of 3.75% form for Actinic Keratoses of the Full Face or Balding Scalp<br><b>Mechanism:</b> TLR Agonist |
| 2011-03-25 | Ipilimumab | Melanoma | Unresectable or Metastatic Melanoma | Locally Advanced or Metastatic | CTLA-4 Ab | CTLA-4 Targeted Antibody | Immunotherapy | 64 | <b>Drug:</b> Ipilimumab | <b>Date of Approval:</b> 2011-03-25 | <b>Indication:</b> Unresectable or Metastatic Melanoma | <b>Mechanism:</b> CTLA-4 Ab | <b>Drug:</b> Ipilimumab<br><b>Date of Approval:</b> 2011-03-25<br><b>Indication:</b> Unresectable or Metastatic Melanoma<br><b>Mechanism:</b> CTLA-4 Ab |
| 2011-03-29 | Peginterferon Alfa-2b | Melanoma | Adjuvant Melanoma | Adjuvant | Cytokine | Cytokine | Immunotherapy | 65 | <b>Drug:</b> Peginterferon Alfa-2b | <b>Date of Approval:</b> 2011-03-29 | <b>Indication:</b> Adjuvant Melanoma | <b>Mechanism:</b> Cytokine | <b>Drug:</b> Peginterferon Alfa-2b<br><b>Date of Approval:</b> 2011-03-29<br><b>Indication:</b> Adjuvant Melanoma<br><b>Mechanism:</b> Cytokine |
| 2011-07-15 | Imiquimod | AK | Use of 2.5% cream for AKs on face and scalp | Other | TLR Agonist | TLR Agonist | Immunotherapy | 66 | <b>Drug:</b> Imiquimod | <b>Date of Approval:</b> 2011-07-15 | <b>Indication:</b> Use of 2.5% cream for AKs on face and scalp | <b>Mechanism:</b> TLR Agonist | <b>Drug:</b> Imiquimod<br><b>Date of Approval:</b> 2011-07-15<br><b>Indication:</b> Use of 2.5% cream for AKs on face and scalp<br><b>Mechanism:</b> TLR Agonist |
| 2011-08-17 | Vemurafenib | Melanoma | Unresectable or Metastatic Melanoma | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 67 | <b>Drug:</b> Vemurafenib | <b>Date of Approval:</b> 2011-08-17 | <b>Indication:</b> Unresectable or Metastatic Melanoma | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Vemurafenib<br><b>Date of Approval:</b> 2011-08-17<br><b>Indication:</b> Unresectable or Metastatic Melanoma<br><b>Mechanism:</b> Kinase Inhibitor |
| 2012-01-23 | Ingenol Mebutate | AK | Actinic Keratoses | Other | Cytotoxic Agent | Cytotoxic Agent | Cytotoxic Agent | 68 | <b>Drug:</b> Ingenol Mebutate | <b>Date of Approval:</b> 2012-01-23 | <b>Indication:</b> Actinic Keratoses | <b>Mechanism:</b> Cytotoxic Agent | <b>Drug:</b> Ingenol Mebutate<br><b>Date of Approval:</b> 2012-01-23<br><b>Indication:</b> Actinic Keratoses<br><b>Mechanism:</b> Cytotoxic Agent |
| 2012-01-30 | Vismodegib | BCC | Locally Advanced or Metastatic Basal Cell Carcinoma | Locally Advanced or Metastatic | Smoothened Inhibitor | Smoothened Inhibitor | Targeted Therapy | 69 | <b>Drug:</b> Vismodegib | <b>Date of Approval:</b> 2012-01-30 | <b>Indication:</b> Locally Advanced or Metastatic Basal Cell Carcinoma | <b>Mechanism:</b> Smoothened Inhibitor | <b>Drug:</b> Vismodegib<br><b>Date of Approval:</b> 2012-01-30<br><b>Indication:</b> Locally Advanced or Metastatic Basal Cell Carcinoma<br><b>Mechanism:</b> Smoothened Inhibitor |
| 2013-05-29 | Trametinib Dimethyl Sulfoxide | Melanoma | Unresectable or Metastatic BRAF-Mutated Melanoma | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 70 | <b>Drug:</b> Trametinib Dimethyl Sulfoxide | <b>Date of Approval:</b> 2013-05-29 | <b>Indication:</b> Unresectable or Metastatic BRAF-Mutated Melanoma | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Trametinib Dimethyl Sulfoxide<br><b>Date of Approval:</b> 2013-05-29<br><b>Indication:</b> Unresectable or Metastatic BRAF-Mutated Melanoma<br><b>Mechanism:</b> Kinase Inhibitor |
| 2013-05-29 | Dabrafenib Mesylate | Melanoma | Single agent, first-line in advanced BRAF-mutated melanoma | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 71 | <b>Drug:</b> Dabrafenib Mesylate | <b>Date of Approval:</b> 2013-05-29 | <b>Indication:</b> Single agent, first-line in advanced BRAF-mutated melanoma | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Dabrafenib Mesylate<br><b>Date of Approval:</b> 2013-05-29<br><b>Indication:</b> Single agent, first-line in advanced BRAF-mutated melanoma<br><b>Mechanism:</b> Kinase Inhibitor |
| 2013-08-23 | Mechlorethamine Hydrochloride | CTCL | Refractory Stage IA and IB Cutaneous T-Cell Lymphoma | Refractory | Alkylating Agent | Alkylating Agent | Cytotoxic Agent | 72 | <b>Drug:</b> Mechlorethamine Hydrochloride | <b>Date of Approval:</b> 2013-08-23 | <b>Indication:</b> Refractory Stage IA and IB Cutaneous T-Cell Lymphoma | <b>Mechanism:</b> Alkylating Agent | <b>Drug:</b> Mechlorethamine Hydrochloride<br><b>Date of Approval:</b> 2013-08-23<br><b>Indication:</b> Refractory Stage IA and IB Cutaneous T-Cell Lymphoma<br><b>Mechanism:</b> Alkylating Agent |
| 2014-01-08 | Trametinib Dimethyl Sulfoxide | Melanoma | Unresectable or Metastatic BRAF-Mutated Melanoma | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 73 | <b>Drug:</b> Trametinib Dimethyl Sulfoxide | <b>Date of Approval:</b> 2014-01-08 | <b>Indication:</b> Unresectable or Metastatic BRAF-Mutated Melanoma | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Trametinib Dimethyl Sulfoxide<br><b>Date of Approval:</b> 2014-01-08<br><b>Indication:</b> Unresectable or Metastatic BRAF-Mutated Melanoma<br><b>Mechanism:</b> Kinase Inhibitor |
| 2014-01-09 | Dabrafenib Mesylate | Melanoma | Accelerated approval for Dab/Tram in advanced BRAF mutant melanoma | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 74 | <b>Drug:</b> Dabrafenib Mesylate | <b>Date of Approval:</b> 2014-01-09 | <b>Indication:</b> Accelerated approval for Dab/Tram in advanced BRAF mutant melanoma | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Dabrafenib Mesylate<br><b>Date of Approval:</b> 2014-01-09<br><b>Indication:</b> Accelerated approval for Dab/Tram in advanced BRAF mutant melanoma<br><b>Mechanism:</b> Kinase Inhibitor |
| 2014-09-04 | Pembrolizumab | Melanoma | Unresectable or Metastatic Melanoma - Second Line | Refractory | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 75 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2014-09-04 | <b>Indication:</b> Unresectable or Metastatic Melanoma - Second Line | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2014-09-04<br><b>Indication:</b> Unresectable or Metastatic Melanoma - Second Line<br><b>Mechanism:</b> PD-1 Ab |
| 2014-12-22 | Nivolumab | Melanoma | Accelerated Approval for Unresectable or Metastatic Melanoma in the Second-Line Setting | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 76 | <b>Drug:</b> Nivolumab | <b>Date of Approval:</b> 2014-12-22 | <b>Indication:</b> Accelerated Approval for Unresectable or Metastatic Melanoma in the Second-Line Setting | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Nivolumab<br><b>Date of Approval:</b> 2014-12-22<br><b>Indication:</b> Accelerated Approval for Unresectable or Metastatic Melanoma in the Second-Line Setting<br><b>Mechanism:</b> PD-1 Ab |
| 2015-07-24 | Sonidegib Phosphate | BCC | Locally Advanced Basal Cell Carcinoma | Locally Advanced | Smoothened Inhibitor | Smoothened Inhibitor | Targeted Therapy | 77 | <b>Drug:</b> Sonidegib Phosphate | <b>Date of Approval:</b> 2015-07-24 | <b>Indication:</b> Locally Advanced Basal Cell Carcinoma | <b>Mechanism:</b> Smoothened Inhibitor | <b>Drug:</b> Sonidegib Phosphate<br><b>Date of Approval:</b> 2015-07-24<br><b>Indication:</b> Locally Advanced Basal Cell Carcinoma<br><b>Mechanism:</b> Smoothened Inhibitor |
| 2015-09-30 | Nivolumab | Melanoma | Accelerated Approval of OPDIVO with YERVOY in BRAF Wild Type Unresectable or Metastatic Melanoma | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 78 | <b>Drug:</b> Nivolumab | <b>Date of Approval:</b> 2015-09-30 | <b>Indication:</b> Accelerated Approval of OPDIVO with YERVOY in BRAF Wild Type Unresectable or Metastatic Melanoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Nivolumab<br><b>Date of Approval:</b> 2015-09-30<br><b>Indication:</b> Accelerated Approval of OPDIVO with YERVOY in BRAF Wild Type Unresectable or Metastatic Melanoma<br><b>Mechanism:</b> PD-1 Ab |
| 2015-10-27 | Talimogene Laherparepvec | Melanoma | Unresectable Cutaneous, Subcutaneous, and Nodal Recurrent Melanoma | Locally Advanced | Oncolytic Virus | Oncolytic Virus | Immunotherapy | 79 | <b>Drug:</b> Talimogene Laherparepvec | <b>Date of Approval:</b> 2015-10-27 | <b>Indication:</b> Unresectable Cutaneous, Subcutaneous, and Nodal Recurrent Melanoma | <b>Mechanism:</b> Oncolytic Virus | <b>Drug:</b> Talimogene Laherparepvec<br><b>Date of Approval:</b> 2015-10-27<br><b>Indication:</b> Unresectable Cutaneous, Subcutaneous, and Nodal Recurrent Melanoma<br><b>Mechanism:</b> Oncolytic Virus |
| 2015-10-28 | Ipilimumab | Melanoma | Adjuvant Melanoma | Adjuvant | CTLA-4 Ab | CTLA-4 Targeted Antibody | Immunotherapy | 80 | <b>Drug:</b> Ipilimumab | <b>Date of Approval:</b> 2015-10-28 | <b>Indication:</b> Adjuvant Melanoma | <b>Mechanism:</b> CTLA-4 Ab | <b>Drug:</b> Ipilimumab<br><b>Date of Approval:</b> 2015-10-28<br><b>Indication:</b> Adjuvant Melanoma<br><b>Mechanism:</b> CTLA-4 Ab |
| 2015-11-10 | Cobimetinib Fumarate | Melanoma | Unresectable or Metastatic Melanoma with a BRAF-Mutated Melanoma In Combination with Vemurafenib | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 81 | <b>Drug:</b> Cobimetinib Fumarate | <b>Date of Approval:</b> 2015-11-10 | <b>Indication:</b> Unresectable or Metastatic Melanoma with a BRAF-Mutated Melanoma In Combination with Vemurafenib | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Cobimetinib Fumarate<br><b>Date of Approval:</b> 2015-11-10<br><b>Indication:</b> Unresectable or Metastatic Melanoma with a BRAF-Mutated Melanoma In Combination with Vemurafenib<br><b>Mechanism:</b> Kinase Inhibitor |
| 2015-11-19 | Ingenol Mebutate | AK | Describing response to a repeat course | Other | Cytotoxic Agent | Cytotoxic Agent | Cytotoxic Agent | 82 | <b>Drug:</b> Ingenol Mebutate | <b>Date of Approval:</b> 2015-11-19 | <b>Indication:</b> Describing response to a repeat course | <b>Mechanism:</b> Cytotoxic Agent | <b>Drug:</b> Ingenol Mebutate<br><b>Date of Approval:</b> 2015-11-19<br><b>Indication:</b> Describing response to a repeat course<br><b>Mechanism:</b> Cytotoxic Agent |
| 2015-11-20 | Trametinib Dimethyl Sulfoxide | Melanoma | Unresectable or Metastatic Melanoma with a BRAF-Mutated Melanoma In Combination with Dabrafenib | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 83 | <b>Drug:</b> Trametinib Dimethyl Sulfoxide | <b>Date of Approval:</b> 2015-11-20 | <b>Indication:</b> Unresectable or Metastatic Melanoma with a BRAF-Mutated Melanoma In Combination with Dabrafenib | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Trametinib Dimethyl Sulfoxide<br><b>Date of Approval:</b> 2015-11-20<br><b>Indication:</b> Unresectable or Metastatic Melanoma with a BRAF-Mutated Melanoma In Combination with Dabrafenib<br><b>Mechanism:</b> Kinase Inhibitor |
| 2015-11-20 | Dabrafenib Mesylate | Melanoma | Regular approval for Dab/Tram in advanced BRAF mutant melanoma | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 84 | <b>Drug:</b> Dabrafenib Mesylate | <b>Date of Approval:</b> 2015-11-20 | <b>Indication:</b> Regular approval for Dab/Tram in advanced BRAF mutant melanoma | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Dabrafenib Mesylate<br><b>Date of Approval:</b> 2015-11-20<br><b>Indication:</b> Regular approval for Dab/Tram in advanced BRAF mutant melanoma<br><b>Mechanism:</b> Kinase Inhibitor |
| 2015-11-23 | Nivolumab | Melanoma | Regular Approval for Unresectable or Metastatic BRAF-Wild Type Melanoma | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 85 | <b>Drug:</b> Nivolumab | <b>Date of Approval:</b> 2015-11-23 | <b>Indication:</b> Regular Approval for Unresectable or Metastatic BRAF-Wild Type Melanoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Nivolumab<br><b>Date of Approval:</b> 2015-11-23<br><b>Indication:</b> Regular Approval for Unresectable or Metastatic BRAF-Wild Type Melanoma<br><b>Mechanism:</b> PD-1 Ab |
| 2015-12-18 | Pembrolizumab | Melanoma | Unresectable or Metastatic Melanoma | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 86 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2015-12-18 | <b>Indication:</b> Unresectable or Metastatic Melanoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2015-12-18<br><b>Indication:</b> Unresectable or Metastatic Melanoma<br><b>Mechanism:</b> PD-1 Ab |
| 2015-12-18 | Pembrolizumab | Melanoma | Removing language limiting the indication to disease progression following ipilimumab | Other | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 87 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2015-12-18 | <b>Indication:</b> Removing language limiting the indication to disease progression following ipilimumab | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2015-12-18<br><b>Indication:</b> Removing language limiting the indication to disease progression following ipilimumab<br><b>Mechanism:</b> PD-1 Ab |
| 2016-01-23 | Nivolumab | Melanoma | Accelerated Approved for BRAF Mutant Unresectable or Metastatic Melanoma in the First Line Setting | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 88 | <b>Drug:</b> Nivolumab | <b>Date of Approval:</b> 2016-01-23 | <b>Indication:</b> Accelerated Approved for BRAF Mutant Unresectable or Metastatic Melanoma in the First Line Setting | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Nivolumab<br><b>Date of Approval:</b> 2016-01-23<br><b>Indication:</b> Accelerated Approved for BRAF Mutant Unresectable or Metastatic Melanoma in the First Line Setting<br><b>Mechanism:</b> PD-1 Ab |
| 2016-01-23 | Nivolumab | Melanoma | Accelerated approval of Ipi/Nivo Expansion for BRAF mutant as well as BRAF WT | Other | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 89 | <b>Drug:</b> Nivolumab | <b>Date of Approval:</b> 2016-01-23 | <b>Indication:</b> Accelerated approval of Ipi/Nivo Expansion for BRAF mutant as well as BRAF WT | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Nivolumab<br><b>Date of Approval:</b> 2016-01-23<br><b>Indication:</b> Accelerated approval of Ipi/Nivo Expansion for BRAF mutant as well as BRAF WT<br><b>Mechanism:</b> PD-1 Ab |
| 2016-05-10 | Aminolevulinic Acid Hydrochloride | AK | Actinic keratoses of mild-to-moderate severity on the face and scalp | Other | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 90 | <b>Drug:</b> Aminolevulinic Acid Hydrochloride | <b>Date of Approval:</b> 2016-05-10 | <b>Indication:</b> Actinic keratoses of mild-to-moderate severity on the face and scalp | <b>Mechanism:</b> Phototoxic | <b>Drug:</b> Aminolevulinic Acid Hydrochloride<br><b>Date of Approval:</b> 2016-05-10<br><b>Indication:</b> Actinic keratoses of mild-to-moderate severity on the face and scalp<br><b>Mechanism:</b> Phototoxic |
| 2016-09-13 | Nivolumab | Melanoma | Flat Dosing for Melanoma, NSCLC and RCC | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 91 | <b>Drug:</b> Nivolumab | <b>Date of Approval:</b> 2016-09-13 | <b>Indication:</b> Flat Dosing for Melanoma, NSCLC and RCC | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Nivolumab<br><b>Date of Approval:</b> 2016-09-13<br><b>Indication:</b> Flat Dosing for Melanoma, NSCLC and RCC<br><b>Mechanism:</b> PD-1 Ab |
| 2016-09-13 | Nivolumab | Melanoma | Flat Dosing for Melanoma, NSCLC and RCC | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 92 | <b>Drug:</b> Nivolumab | <b>Date of Approval:</b> 2016-09-13 | <b>Indication:</b> Flat Dosing for Melanoma, NSCLC and RCC | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Nivolumab<br><b>Date of Approval:</b> 2016-09-13<br><b>Indication:</b> Flat Dosing for Melanoma, NSCLC and RCC<br><b>Mechanism:</b> PD-1 Ab |
| 2017-03-23 | Avelumab | MCC | Metastatic Merkel Cell Carcinoma | Metastatic | PD-L1 Ab | PD-L1 Targeted Antibody | Immunotherapy | 93 | <b>Drug:</b> Avelumab | <b>Date of Approval:</b> 2017-03-23 | <b>Indication:</b> Metastatic Merkel Cell Carcinoma | <b>Mechanism:</b> PD-L1 Ab | <b>Drug:</b> Avelumab<br><b>Date of Approval:</b> 2017-03-23<br><b>Indication:</b> Metastatic Merkel Cell Carcinoma<br><b>Mechanism:</b> PD-L1 Ab |
| 2017-05-17 | Pembrolizumab | Melanoma | Flat Dosing of 200 mg q3 weeks in melanoma | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 94 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2017-05-17 | <b>Indication:</b> Flat Dosing of 200 mg q3 weeks in melanoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2017-05-17<br><b>Indication:</b> Flat Dosing of 200 mg q3 weeks in melanoma<br><b>Mechanism:</b> PD-1 Ab |
| 2017-05-23 | Pembrolizumab | Agnostic | Unresectable or Metastatic, Microsatellite Instability-High or Mismatch Repair Deficient Solid Tumors | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 95 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2017-05-23 | <b>Indication:</b> Unresectable or Metastatic, Microsatellite Instability-High or Mismatch Repair Deficient Solid Tumors | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2017-05-23<br><b>Indication:</b> Unresectable or Metastatic, Microsatellite Instability-High or Mismatch Repair Deficient Solid Tumors<br><b>Mechanism:</b> PD-1 Ab |
| 2017-07-21 | Ipilimumab | Melanoma | Unresectable or Metastatic Melanoma - patients 12 years and older | Locally Advanced or Metastatic | CTLA-4 Ab | CTLA-4 Targeted Antibody | Immunotherapy | 96 | <b>Drug:</b> Ipilimumab | <b>Date of Approval:</b> 2017-07-21 | <b>Indication:</b> Unresectable or Metastatic Melanoma - patients 12 years and older | <b>Mechanism:</b> CTLA-4 Ab | <b>Drug:</b> Ipilimumab<br><b>Date of Approval:</b> 2017-07-21<br><b>Indication:</b> Unresectable or Metastatic Melanoma - patients 12 years and older<br><b>Mechanism:</b> CTLA-4 Ab |
| 2017-11-09 | Brentuximab Vedotin | CTCL | CD30+ MF and pcALCL in the second line | Refractory | CD30 Ab | CD30 Targeted Antibody | Targeted Therapy | 97 | <b>Drug:</b> Brentuximab Vedotin | <b>Date of Approval:</b> 2017-11-09 | <b>Indication:</b> CD30+ MF and pcALCL in the second line | <b>Mechanism:</b> CD30 Ab | <b>Drug:</b> Brentuximab Vedotin<br><b>Date of Approval:</b> 2017-11-09<br><b>Indication:</b> CD30+ MF and pcALCL in the second line<br><b>Mechanism:</b> CD30 Ab |
| 2017-12-20 | Nivolumab | Melanoma | Regular Approval for Adjuvant Melanoma | Adjuvant | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 98 | <b>Drug:</b> Nivolumab | <b>Date of Approval:</b> 2017-12-20 | <b>Indication:</b> Regular Approval for Adjuvant Melanoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Nivolumab<br><b>Date of Approval:</b> 2017-12-20<br><b>Indication:</b> Regular Approval for Adjuvant Melanoma<br><b>Mechanism:</b> PD-1 Ab |
| 2018-01-09 | Nivolumab | Melanoma | Updates infusion from 60 to 30 minutes | Other | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 99 | <b>Drug:</b> Nivolumab | <b>Date of Approval:</b> 2018-01-09 | <b>Indication:</b> Updates infusion from 60 to 30 minutes | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Nivolumab<br><b>Date of Approval:</b> 2018-01-09<br><b>Indication:</b> Updates infusion from 60 to 30 minutes<br><b>Mechanism:</b> PD-1 Ab |
| 2018-01-26 | Cobimetinib Fumarate | Melanoma | Updates label to include OS data | Other | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 100 | <b>Drug:</b> Cobimetinib Fumarate | <b>Date of Approval:</b> 2018-01-26 | <b>Indication:</b> Updates label to include OS data | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Cobimetinib Fumarate<br><b>Date of Approval:</b> 2018-01-26<br><b>Indication:</b> Updates label to include OS data<br><b>Mechanism:</b> Kinase Inhibitor |
| 2018-03-05 | Nivolumab | Melanoma | Every 4 Week Dosing for Advanced Melanoma | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 101 | <b>Drug:</b> Nivolumab | <b>Date of Approval:</b> 2018-03-05 | <b>Indication:</b> Every 4 Week Dosing for Advanced Melanoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Nivolumab<br><b>Date of Approval:</b> 2018-03-05<br><b>Indication:</b> Every 4 Week Dosing for Advanced Melanoma<br><b>Mechanism:</b> PD-1 Ab |
| 2018-03-05 | Nivolumab | Melanoma | Every 4 week dosing in Adjuvant Melanoma | Adjuvant | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 102 | <b>Drug:</b> Nivolumab | <b>Date of Approval:</b> 2018-03-05 | <b>Indication:</b> Every 4 week dosing in Adjuvant Melanoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Nivolumab<br><b>Date of Approval:</b> 2018-03-05<br><b>Indication:</b> Every 4 week dosing in Adjuvant Melanoma<br><b>Mechanism:</b> PD-1 Ab |
| 2018-03-05 | Nivolumab | Melanoma | Updates infusion from 60 to 30 minutes in adjuvant Melanoma | Adjuvant | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 103 | <b>Drug:</b> Nivolumab | <b>Date of Approval:</b> 2018-03-05 | <b>Indication:</b> Updates infusion from 60 to 30 minutes in adjuvant Melanoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Nivolumab<br><b>Date of Approval:</b> 2018-03-05<br><b>Indication:</b> Updates infusion from 60 to 30 minutes in adjuvant Melanoma<br><b>Mechanism:</b> PD-1 Ab |
| 2018-03-06 | Aminolevulinic Acid Hydrochloride | AK | Minimally to moderately thick Actinic Keratosis of the upper extremities | Other | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 104 | <b>Drug:</b> Aminolevulinic Acid Hydrochloride | <b>Date of Approval:</b> 2018-03-06 | <b>Indication:</b> Minimally to moderately thick Actinic Keratosis of the upper extremities | <b>Mechanism:</b> Phototoxic | <b>Drug:</b> Aminolevulinic Acid Hydrochloride<br><b>Date of Approval:</b> 2018-03-06<br><b>Indication:</b> Minimally to moderately thick Actinic Keratosis of the upper extremities<br><b>Mechanism:</b> Phototoxic |
| 2018-04-30 | Trametinib Dimethyl Sulfoxide | Melanoma | Adjuvant BRAF Mutant Melanoma In Combination with Dabrafenib | Adjuvant | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 105 | <b>Drug:</b> Trametinib Dimethyl Sulfoxide | <b>Date of Approval:</b> 2018-04-30 | <b>Indication:</b> Adjuvant BRAF Mutant Melanoma In Combination with Dabrafenib | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Trametinib Dimethyl Sulfoxide<br><b>Date of Approval:</b> 2018-04-30<br><b>Indication:</b> Adjuvant BRAF Mutant Melanoma In Combination with Dabrafenib<br><b>Mechanism:</b> Kinase Inhibitor |
| 2018-04-30 | Dabrafenib Mesylate | Melanoma | Adjuvant BRAF Mutant Melanoma In Combination with Trametinib | Adjuvant | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 106 | <b>Drug:</b> Dabrafenib Mesylate | <b>Date of Approval:</b> 2018-04-30 | <b>Indication:</b> Adjuvant BRAF Mutant Melanoma In Combination with Trametinib | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Dabrafenib Mesylate<br><b>Date of Approval:</b> 2018-04-30<br><b>Indication:</b> Adjuvant BRAF Mutant Melanoma In Combination with Trametinib<br><b>Mechanism:</b> Kinase Inhibitor |
| 2018-06-27 | Encorafenib | Melanoma | Regular Approval for use in combination with binimetinib for advanced BRAF mutant melanoma | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 107 | <b>Drug:</b> Encorafenib | <b>Date of Approval:</b> 2018-06-27 | <b>Indication:</b> Regular Approval for use in combination with binimetinib for advanced BRAF mutant melanoma | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Encorafenib<br><b>Date of Approval:</b> 2018-06-27<br><b>Indication:</b> Regular Approval for use in combination with binimetinib for advanced BRAF mutant melanoma<br><b>Mechanism:</b> Kinase Inhibitor |
| 2018-06-27 | Binimetinib | Melanoma | Unresectable or Metastatic BRAF-Mutated Melanoma In Combination with Encorafenib | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 108 | <b>Drug:</b> Binimetinib | <b>Date of Approval:</b> 2018-06-27 | <b>Indication:</b> Unresectable or Metastatic BRAF-Mutated Melanoma In Combination with Encorafenib | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Binimetinib<br><b>Date of Approval:</b> 2018-06-27<br><b>Indication:</b> Unresectable or Metastatic BRAF-Mutated Melanoma In Combination with Encorafenib<br><b>Mechanism:</b> Kinase Inhibitor |
| 2018-08-08 | Mogamulizumab-Kpkc | CTCL | Relapsed/Refractory Mycosis Fungoides or Sezary Syndrome - Second Line | Refractory | CCR4 Ab | CCR4 Targeted Antibody & ADCC Inducer | Immunotherapy | 109 | <b>Drug:</b> Mogamulizumab-Kpkc | <b>Date of Approval:</b> 2018-08-08 | <b>Indication:</b> Relapsed/Refractory Mycosis Fungoides or Sezary Syndrome - Second Line | <b>Mechanism:</b> CCR4 Ab | <b>Drug:</b> Mogamulizumab-Kpkc<br><b>Date of Approval:</b> 2018-08-08<br><b>Indication:</b> Relapsed/Refractory Mycosis Fungoides or Sezary Syndrome - Second Line<br><b>Mechanism:</b> CCR4 Ab |
| 2018-09-28 | Cemiplimab-Rwlc | SCC | Locally Advanced or Metastatic Squamous Cell Carcinoma | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 110 | <b>Drug:</b> Cemiplimab-Rwlc | <b>Date of Approval:</b> 2018-09-28 | <b>Indication:</b> Locally Advanced or Metastatic Squamous Cell Carcinoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Cemiplimab-Rwlc<br><b>Date of Approval:</b> 2018-09-28<br><b>Indication:</b> Locally Advanced or Metastatic Squamous Cell Carcinoma<br><b>Mechanism:</b> PD-1 Ab |
| 2018-12-19 | Pembrolizumab | MCC | Locally Advanced or Metastatic Merkel Cell Carcinoma | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 111 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2018-12-19 | <b>Indication:</b> Locally Advanced or Metastatic Merkel Cell Carcinoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2018-12-19<br><b>Indication:</b> Locally Advanced or Metastatic Merkel Cell Carcinoma<br><b>Mechanism:</b> PD-1 Ab |
| 2019-01-18 | Vismodegib | BCC | To allow for treatment interruptions of up to 8 weeks for intolerable AEs | Other | Smoothened Inhibitor | Smoothened Inhibitor | Targeted Therapy | 112 | <b>Drug:</b> Vismodegib | <b>Date of Approval:</b> 2019-01-18 | <b>Indication:</b> To allow for treatment interruptions of up to 8 weeks for intolerable AEs | <b>Mechanism:</b> Smoothened Inhibitor | <b>Drug:</b> Vismodegib<br><b>Date of Approval:</b> 2019-01-18<br><b>Indication:</b> To allow for treatment interruptions of up to 8 weeks for intolerable AEs<br><b>Mechanism:</b> Smoothened Inhibitor |
| 2019-02-15 | Pembrolizumab | Melanoma | Adjuvant Melanoma | Adjuvant | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 113 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2019-02-15 | <b>Indication:</b> Adjuvant Melanoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2019-02-15<br><b>Indication:</b> Adjuvant Melanoma<br><b>Mechanism:</b> PD-1 Ab |
| 2019-03-07 | Nivolumab | Melanoma | Conversion to Regular Approval for the treatment of patients with BRAF V600 mutation-positive unresectable or metastatic melanoma | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 114 | <b>Drug:</b> Nivolumab | <b>Date of Approval:</b> 2019-03-07 | <b>Indication:</b> Conversion to Regular Approval for the treatment of patients with BRAF V600 mutation-positive unresectable or metastatic melanoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Nivolumab<br><b>Date of Approval:</b> 2019-03-07<br><b>Indication:</b> Conversion to Regular Approval for the treatment of patients with BRAF V600 mutation-positive unresectable or metastatic melanoma<br><b>Mechanism:</b> PD-1 Ab |
| 2019-03-07 | Nivolumab | Melanoma | Conversion to Regular Approval of OPDIVO, in combination with ipilimumab, for the treatment of patients with unresectable or metastatic melanoma | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 115 | <b>Drug:</b> Nivolumab | <b>Date of Approval:</b> 2019-03-07 | <b>Indication:</b> Conversion to Regular Approval of OPDIVO, in combination with ipilimumab, for the treatment of patients with unresectable or metastatic melanoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Nivolumab<br><b>Date of Approval:</b> 2019-03-07<br><b>Indication:</b> Conversion to Regular Approval of OPDIVO, in combination with ipilimumab, for the treatment of patients with unresectable or metastatic melanoma<br><b>Mechanism:</b> PD-1 Ab |
| 2019-10-06 | Trametinib Dimethyl Sulfoxide | Melanoma | Updating results in subjects with brain metastases | Other | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 116 | <b>Drug:</b> Trametinib Dimethyl Sulfoxide | <b>Date of Approval:</b> 2019-10-06 | <b>Indication:</b> Updating results in subjects with brain metastases | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Trametinib Dimethyl Sulfoxide<br><b>Date of Approval:</b> 2019-10-06<br><b>Indication:</b> Updating results in subjects with brain metastases<br><b>Mechanism:</b> Kinase Inhibitor |
| 2019-10-06 | Dabrafenib Mesylate | Melanoma | Updates label in regards to brain mets for melanoma | Other | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 117 | <b>Drug:</b> Dabrafenib Mesylate | <b>Date of Approval:</b> 2019-10-06 | <b>Indication:</b> Updates label in regards to brain mets for melanoma | <b>Mechanism:</b> Kinase Inhibitor | <b>Drug:</b> Dabrafenib Mesylate<br><b>Date of Approval:</b> 2019-10-06<br><b>Indication:</b> Updates label in regards to brain mets for melanoma<br><b>Mechanism:</b> Kinase Inhibitor |
| 2020-04-28 | Pembrolizumab | Melanoma | Every 6 weeks dosing in Melanoma | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 118 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2020-04-28 | <b>Indication:</b> Every 6 weeks dosing in Melanoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2020-04-28<br><b>Indication:</b> Every 6 weeks dosing in Melanoma<br><b>Mechanism:</b> PD-1 Ab |
| 2020-04-28 | Pembrolizumab | MCC | Every 6 weeks dosing in Merkel Cell Carcinoma | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 119 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2020-04-28 | <b>Indication:</b> Every 6 weeks dosing in Merkel Cell Carcinoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2020-04-28<br><b>Indication:</b> Every 6 weeks dosing in Merkel Cell Carcinoma<br><b>Mechanism:</b> PD-1 Ab |
| 2020-04-28 | Pembrolizumab | Agnostic | Every 6 weeks dosing in MSI-H tumors | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 120 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2020-04-28 | <b>Indication:</b> Every 6 weeks dosing in MSI-H tumors | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2020-04-28<br><b>Indication:</b> Every 6 weeks dosing in MSI-H tumors<br><b>Mechanism:</b> PD-1 Ab |
| 2020-05-14 | Pomalidomide | KS | Treatment of adult patients with AIDS-related Kaposi sarcoma | Other | Immunomodulatory | Cereblon Inhibitor | Imid | 121 | <b>Drug:</b> Pomalidomide | <b>Date of Approval:</b> 2020-05-14 | <b>Indication:</b> Treatment of adult patients with AIDS-related Kaposi sarcoma | <b>Mechanism:</b> Immunomodulatory | <b>Drug:</b> Pomalidomide<br><b>Date of Approval:</b> 2020-05-14<br><b>Indication:</b> Treatment of adult patients with AIDS-related Kaposi sarcoma<br><b>Mechanism:</b> Immunomodulatory |
| 2020-05-14 | Pomalidomide | KS | Treatment of Kaposi’s Sarcoma in patients who are HIV-negative | Other | Immunomodulatory | Cereblon Inhibitor | Imid | 122 | <b>Drug:</b> Pomalidomide | <b>Date of Approval:</b> 2020-05-14 | <b>Indication:</b> Treatment of Kaposi’s Sarcoma in patients who are HIV-negative | <b>Mechanism:</b> Immunomodulatory | <b>Drug:</b> Pomalidomide<br><b>Date of Approval:</b> 2020-05-14<br><b>Indication:</b> Treatment of Kaposi’s Sarcoma in patients who are HIV-negative<br><b>Mechanism:</b> Immunomodulatory |
| 2020-06-16 | Pembrolizumab | Agnostic | Tumor Mutation Burden-High solid tumors | Other | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 123 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2020-06-16 | <b>Indication:</b> Tumor Mutation Burden-High solid tumors | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2020-06-16<br><b>Indication:</b> Tumor Mutation Burden-High solid tumors<br><b>Mechanism:</b> PD-1 Ab |
| 2020-06-16 | Pembrolizumab | Agnostic | Every 6 weeks dosing in TMB-H tumors | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 124 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2020-06-16 | <b>Indication:</b> Every 6 weeks dosing in TMB-H tumors | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2020-06-16<br><b>Indication:</b> Every 6 weeks dosing in TMB-H tumors<br><b>Mechanism:</b> PD-1 Ab |
| 2020-06-24 | Pembrolizumab | SCC | r/mCSCC | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 125 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2020-06-24 | <b>Indication:</b> r/mCSCC | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2020-06-24<br><b>Indication:</b> r/mCSCC<br><b>Mechanism:</b> PD-1 Ab |
| 2020-06-24 | Pembrolizumab | SCC | Every 6 weeks dosing for CSCC | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 126 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2020-06-24 | <b>Indication:</b> Every 6 weeks dosing for CSCC | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2020-06-24<br><b>Indication:</b> Every 6 weeks dosing for CSCC<br><b>Mechanism:</b> PD-1 Ab |
| 2020-07-30 | Atezolizumab | Melanoma | Unresectable or metastatic BRAF-mutated melanoma in combination with cobimetinib and vemurafenib | Locally Advanced or Metastatic | PD-L1 Ab | PD-L1 Targeted Antibody | Immunotherapy | 127 | <b>Drug:</b> Atezolizumab | <b>Date of Approval:</b> 2020-07-30 | <b>Indication:</b> Unresectable or metastatic BRAF-mutated melanoma in combination with cobimetinib and vemurafenib | <b>Mechanism:</b> PD-L1 Ab | <b>Drug:</b> Atezolizumab<br><b>Date of Approval:</b> 2020-07-30<br><b>Indication:</b> Unresectable or metastatic BRAF-mutated melanoma in combination with cobimetinib and vemurafenib<br><b>Mechanism:</b> PD-L1 Ab |
| 2020-12-14 | Tirbanibulin | AK | Actinic keratosis of the face or scalp | Other | Microtubule Inhibitor | Microtubule Inhibitor | Cytotoxic Agent | 128 | <b>Drug:</b> Tirbanibulin | <b>Date of Approval:</b> 2020-12-14 | <b>Indication:</b> Actinic keratosis of the face or scalp | <b>Mechanism:</b> Microtubule Inhibitor | <b>Drug:</b> Tirbanibulin<br><b>Date of Approval:</b> 2020-12-14<br><b>Indication:</b> Actinic keratosis of the face or scalp<br><b>Mechanism:</b> Microtubule Inhibitor |
| 2021-02-09 | Cemiplimab-Rwlc | BCC | Locally Advanced Basal Cell Carcinoma | Locally Advanced | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 129 | <b>Drug:</b> Cemiplimab-Rwlc | <b>Date of Approval:</b> 2021-02-09 | <b>Indication:</b> Locally Advanced Basal Cell Carcinoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Cemiplimab-Rwlc<br><b>Date of Approval:</b> 2021-02-09<br><b>Indication:</b> Locally Advanced Basal Cell Carcinoma<br><b>Mechanism:</b> PD-1 Ab |
| 2021-02-09 | Cemiplimab-Rwlc | BCC | Metastatic Basal Cell Carcinoma | Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 130 | <b>Drug:</b> Cemiplimab-Rwlc | <b>Date of Approval:</b> 2021-02-09 | <b>Indication:</b> Metastatic Basal Cell Carcinoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Cemiplimab-Rwlc<br><b>Date of Approval:</b> 2021-02-09<br><b>Indication:</b> Metastatic Basal Cell Carcinoma<br><b>Mechanism:</b> PD-1 Ab |
| 2021-07-01 | Pembrolizumab | SCC | laCSCC | Locally Advanced | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 131 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2021-07-01 | <b>Indication:</b> laCSCC | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2021-07-01<br><b>Indication:</b> laCSCC<br><b>Mechanism:</b> PD-1 Ab |
| 2021-12-03 | Pembrolizumab | Melanoma | Adjuvant Stage IIB and IIC Melanoma | Adjuvant | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 132 | <b>Drug:</b> Pembrolizumab | <b>Date of Approval:</b> 2021-12-03 | <b>Indication:</b> Adjuvant Stage IIB and IIC Melanoma | <b>Mechanism:</b> PD-1 Ab | <b>Drug:</b> Pembrolizumab<br><b>Date of Approval:</b> 2021-12-03<br><b>Indication:</b> Adjuvant Stage IIB and IIC Melanoma<br><b>Mechanism:</b> PD-1 Ab |
| 2022-01-25 | Tebentafusp-Tebn | Melanoma | HLA-A*02:01-positive adult patients with unresectable or metastatic uveal melanoma | Locally Advanced or Metastatic | Bispecific Antibody | Bispecific gp100 peptide-HLA-A*02:01 directed T cell receptor CD3 T cell engager | Immunotherapy | 133 | <b>Drug:</b> Tebentafusp-Tebn | <b>Date of Approval:</b> 2022-01-25 | <b>Indication:</b> HLA-A*02:01-positive adult patients with unresectable or metastatic uveal melanoma | <b>Mechanism:</b> Bispecific Antibody | <b>Drug:</b> Tebentafusp-Tebn<br><b>Date of Approval:</b> 2022-01-25<br><b>Indication:</b> HLA-A*02:01-positive adult patients with unresectable or metastatic uveal melanoma<br><b>Mechanism:</b> Bispecific Antibody |
| 2022-03-18 | Relatlimab-Rmbw | Melanoma | Unresectable or Metastatic Melanoma | Locally Advanced or Metastatic | LAG-3 Ab | LAG-3 Targeted Antibody | Immunotherapy | 134 | <b>Drug:</b> Relatlimab-Rmbw | <b>Date of Approval:</b> 2022-03-18 | <b>Indication:</b> Unresectable or Metastatic Melanoma | <b>Mechanism:</b> LAG-3 Ab | <b>Drug:</b> Relatlimab-Rmbw<br><b>Date of Approval:</b> 2022-03-18<br><b>Indication:</b> Unresectable or Metastatic Melanoma<br><b>Mechanism:</b> LAG-3 Ab |
skincancerRx_data |>
filter(Dz == "Melanoma") |>
fda_approval_timeliner_df() |>
skincancerRx::fda_approval_timeliner_plot(
.title = "<b>FDA Approvals for Melanoma<b>",
.startbuffer = 1000,
.endbuffer = 1400,
.geomtextsize = 4,
.legendtextsize = 2,
.hoverlabeltextsize = 14,
.legend = FALSE,
.xaxistickfontsize = 14
)fda_approval_timeseries_df() |>
kable() %>%
kable_styling(bootstrap_options = c("striped","hover"),
fixed_thead = T) %>%
scroll_box(height = "400px",
width = "100%")| date | name | Dz | Indication_brief | Indication_short | Mechanism | Mechanism_long | Mechanistic_Class | y |
|---|---|---|---|---|---|---|---|---|
| 1949-03-15 | MECHLORETHAMINE HYDROCHLORIDE | CTCL | Mycosis Fungoides | Other | Alkylating Agent | Alkylating Agent | Cytotoxic Agent | 1 |
| 1950-06-13 | CORTISONE ACETATE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 2 |
| 1950-12-04 | CORTISONE ACETATE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 3 |
| 1952-12-15 | HYDROCORTISONE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 4 |
| 1953-12-07 | METHOTREXATE SODIUM | CTCL | Mycosis Fungoides | Other | Antimetabolite | Antimetabolite | Cytotoxic Agent | 5 |
| 1955-02-21 | PREDNISONE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 6 |
| 1955-04-27 | HYDROCORTISONE SODIUM SUCCINATE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 7 |
| 1955-06-21 | PREDNISOLONE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 8 |
| 1957-10-24 | METHYLPREDNISOLONE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 9 |
| 1957-12-03 | TRIAMCINOLONE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 10 |
| 1958-10-30 | DEXAMETHASONE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 11 |
| 1959-03-12 | TRIAMCINOLONE DIACETATE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 12 |
| 1959-05-18 | METHYLPREDNISOLONE SODIUM SUCCINATE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 13 |
| 1959-05-27 | METHYLPREDNISOLONE ACETATE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 14 |
| 1959-08-10 | METHOTREXATE SODIUM | CTCL | Mycosis Fungoides | Other | Antimetabolite | Antimetabolite | Cytotoxic Agent | 15 |
| 1959-10-06 | DEXAMETHASONE SODIUM PHOSPHATE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 16 |
| 1959-11-16 | CYCLOPHOSPHAMIDE | CTCL | Mycosis Fungoides | Other | Alkylating Agent | Alkylating Agent | Cytotoxic Agent | 17 |
| 1959-11-16 | CYCLOPHOSPHAMIDE | CTCL | Mycosis Fungoides | Other | Alkylating Agent | Alkylating Agent | Cytotoxic Agent | 18 |
| 1960-06-21 | METHYLPREDNISOLONE ACETATE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 19 |
| 1960-07-07 | DEXAMETHASONE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 20 |
| 1961-04-17 | BETAMETHASONE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 21 |
| 1961-09-05 | TRIAMCINOLONE DIACETATE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 22 |
| 1965-02-01 | TRIAMCINOLONE ACETONIDE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 23 |
| 1965-03-03 | BETAMETHASONE ACETATE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 24 |
| 1965-11-25 | VINBLASTINE SULFATE | CTCL | Mycosis Fungoides | Other | Microtubule Inhibitor | Microtubule Inhibitor | Cytotoxic Agent | 25 |
| 1965-11-25 | VINBLASTINE SULFATE | KS | Kaposi's Sarcoma | Other | Microtubule Inhibitor | Microtubule Inhibitor | Cytotoxic Agent | 26 |
| 1967-12-07 | HYDROXYUREA | Melanoma | Melanoma | Other | Antimetabolite | Antimetabolite | Cytotoxic Agent | 27 |
| 1970-07-29 | FLUOROURACIL | AK | Multiple Actinic Keratoses | Other | Antimetabolite | Antimetabolite | Cytotoxic Agent | 28 |
| 1971-08-06 | FLUOROURACIL | AK | Actinic Keratoses | Other | Antimetabolite | Antimetabolite | Cytotoxic Agent | 29 |
| 1975-05-27 | DACARBAZINE | Melanoma | Metastatic Malignant Melanoma | Metastatic | Alkylating Agent | Alkylating Agent | Cytotoxic Agent | 30 |
| 1975-06-30 | FLUOROURACIL | BCC | Superficial Basal Cell Carcinoma | Other | Antimetabolite | Antimetabolite | Cytotoxic Agent | 31 |
| 1986-05-28 | PREDNISOLONE SODIUM PHOSPHATE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 32 |
| 1988-03-23 | METHOXSALEN | CTCL | Refractory Cutaneous T-Cell Lymphoma | Refractory | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 33 |
| 1988-11-21 | INTERFERON ALFA-2B | KS | AIDS-Related Kaposi's Sarcoma | Other | Cytokine | Cytokine | Immunotherapy | 34 |
| 1988-11-21 | INTERFERON ALFA-2A | KS | AIDS-Related Kaposi's Sarcoma | Other | Cytokine | Cytokine | Immunotherapy | 35 |
| 1995-11-17 | DOXORUBICIN HYDROCHLORIDE | KS | Accelerated Approval for Refractory AIDS-Related Kaposi's Sarcoma | Refractory | Topoisomerase Inhibitor | Topoisomerase Inhibitor | Cytotoxic Agent | 36 |
| 1995-12-05 | INTERFERON ALFA-2B | Melanoma | Adjuvant Melanoma | Adjuvant | Cytokine | Cytokine | Immunotherapy | 37 |
| 1996-04-08 | DAUNORUBICIN CITRATE | KS | HIV-associated Kaposi's Sarcoma - First Line | Other | Topoisomerase Inhibitor | Topoisomerase Inhibitor | Cytotoxic Agent | 38 |
| 1997-08-04 | PACLITAXEL | KS | AIDS-Related Kaposi's Sarcoma - Second Line | Refractory | Microtubule Stabilizer | Microtubule Stabilizer | Cytotoxic Agent | 39 |
| 1998-01-09 | ALDESLEUKIN | Melanoma | Metastatic Melanoma | Metastatic | Cytokine | Cytokine | Immunotherapy | 40 |
| 1998-12-17 | PREDNISOLONE SODIUM PHOSPHATE | CTCL | Expansion to include pediatric populations | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 41 |
| 1999-02-02 | ALITRETINOIN | KS | AIDS-Related Kaposi's Sarcoma - Cutaneous Lesions Only | Other | Retinoid | Retinoid | Retinoid | 42 |
| 1999-02-05 | DENILEUKIN DIFTITOX | CTCL | Accelerated Approval for Persistent or Recurrent CD25+ Cutaneous T-Cell Lymphoma | Other | Cytokine-Cytotoxin | Cytokine-Cytotoxin | Cytotoxic Agent | 43 |
| 1999-02-25 | METHOXSALEN | CTCL | Refractory Cutaneous T-Cell Lymphoma | Refractory | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 44 |
| 1999-12-03 | AMINOLEVULINIC ACID HYDROCHLORIDE | AK | Non-Hyperkeratotic Actinic Keratoses of the Face or Scalp | Other | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 45 |
| 1999-12-29 | BEXAROTENE | CTCL | Refractory Cutaneous T-Cell Lymphoma | Refractory | Retinoid | Retinoid | Retinoid | 46 |
| 2000-06-28 | BEXAROTENE | CTCL | Refractory Stage IA and IB Cutaneous T-Cell Lymphoma | Refractory | Retinoid | Retinoid | Retinoid | 47 |
| 2000-10-16 | DICLOFENAC SODIUM | AK | Actinic Keratoses | Other | NSAID | NSAID | NSAID | 48 |
| 2000-10-27 | FLUOROURACIL | AK | Multiple Actinic Keratoses of the Face and Anterior Scalp | Other | Antimetabolite | Antimetabolite | Cytotoxic Agent | 49 |
| 2004-03-02 | IMIQUIMOD | AK | Nonhypertrophic Actinic Keratoses on the Face or Scalp | Other | TLR Agonist | TLR Agonist | Immunotherapy | 50 |
| 2004-07-14 | IMIQUIMOD | BCC | Superficial Basal Cell Carcinoma | Other | TLR Agonist | TLR Agonist | Immunotherapy | 51 |
| 2004-07-27 | METHYL AMINOLEVULINATE HYDROCHLORIDE | AK | Non-Keratotic Actinic Keratoses of the Face and Scalp | Other | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 52 |
| 2006-06-01 | PREDNISOLONE SODIUM PHOSPHATE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 53 |
| 2006-10-06 | VORINOSTAT | CTCL | Refractory Cutaneous T-Cell Lymphoma | Refractory | HDAC Inhibitor | HDAC Inhibitor | Targeted Therapy | 54 |
| 2006-10-19 | IMATINIB MESYLATE | DFSP | Unresectable, Recurrent and/or Metastatic Dermatofibrosarcoma Protuberans | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 55 |
| 2008-01-17 | PREDNISOLONE ACETATE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 56 |
| 2008-06-10 | DOXORUBICIN HYDROCHLORIDE | KS | Regular Aprroval for Refractory AIDS-Related Kaposi's Sarcoma | Refractory | Topoisomerase Inhibitor | Topoisomerase Inhibitor | Cytotoxic Agent | 57 |
| 2008-06-16 | TRIAMCINOLONE ACETONIDE | CTCL | Mycosis Fungoides | Other | Glucocorticoid | Glucocorticoid | Glucocorticoid | 58 |
| 2008-06-26 | METHYL AMINOLEVULINATE HYDROCHLORIDE | AK | Use of Metvixia with a new Lamp (Aktilite) in AKs | Other | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 59 |
| 2008-10-15 | DENILEUKIN DIFTITOX | CTCL | Regular Approval for Persistent or Recurrent CD25+ Cutaneous T-Cell Lymphoma | Other | Cytokine-Cytotoxin | Cytokine-Cytotoxin | Cytotoxic Agent | 60 |
| 2009-11-05 | ROMIDEPSIN | CTCL | Refractory Cutaneous T-Cell Lymphoma | Refractory | HDAC Inhibitor | HDAC Inhibitor | HDAC Inhibitor | 61 |
| 2010-03-12 | AMINOLEVULINIC ACID HYDROCHLORIDE | AK | Allow user to mix contents for 30 seconds prior to use & to use 'Kerastick Krusher' | Other | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 62 |
| 2010-03-25 | IMIQUIMOD | AK | Use of 3.75% form for Actinic Keratoses of the Full Face or Balding Scalp | Other | TLR Agonist | TLR Agonist | Immunotherapy | 63 |
| 2011-03-25 | IPILIMUMAB | Melanoma | Unresectable or Metastatic Melanoma | Locally Advanced or Metastatic | CTLA-4 Ab | CTLA-4 Targeted Antibody | Immunotherapy | 64 |
| 2011-03-29 | PEGINTERFERON ALFA-2B | Melanoma | Adjuvant Melanoma | Adjuvant | Cytokine | Cytokine | Immunotherapy | 65 |
| 2011-07-15 | IMIQUIMOD | AK | Use of 2.5% cream for AKs on face and scalp | Other | TLR Agonist | TLR Agonist | Immunotherapy | 66 |
| 2011-08-17 | VEMURAFENIB | Melanoma | Unresectable or Metastatic Melanoma | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 67 |
| 2012-01-23 | INGENOL MEBUTATE | AK | Actinic Keratoses | Other | Cytotoxic Agent | Cytotoxic Agent | Cytotoxic Agent | 68 |
| 2012-01-30 | VISMODEGIB | BCC | Locally Advanced or Metastatic Basal Cell Carcinoma | Locally Advanced or Metastatic | Smoothened Inhibitor | Smoothened Inhibitor | Targeted Therapy | 69 |
| 2013-05-29 | TRAMETINIB DIMETHYL SULFOXIDE | Melanoma | Unresectable or Metastatic BRAF-Mutated Melanoma | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 70 |
| 2013-05-29 | DABRAFENIB MESYLATE | Melanoma | Single agent, first-line in advanced BRAF-mutated melanoma | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 71 |
| 2013-08-23 | MECHLORETHAMINE HYDROCHLORIDE | CTCL | Refractory Stage IA and IB Cutaneous T-Cell Lymphoma | Refractory | Alkylating Agent | Alkylating Agent | Cytotoxic Agent | 72 |
| 2014-01-08 | TRAMETINIB DIMETHYL SULFOXIDE | Melanoma | Unresectable or Metastatic BRAF-Mutated Melanoma | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 73 |
| 2014-01-09 | DABRAFENIB MESYLATE | Melanoma | Accelerated approval for Dab/Tram in advanced BRAF mutant melanoma | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 74 |
| 2014-09-04 | PEMBROLIZUMAB | Melanoma | Unresectable or Metastatic Melanoma - Second Line | Refractory | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 75 |
| 2014-12-22 | NIVOLUMAB | Melanoma | Accelerated Approval for Unresectable or Metastatic Melanoma in the Second-Line Setting | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 76 |
| 2015-07-24 | SONIDEGIB PHOSPHATE | BCC | Locally Advanced Basal Cell Carcinoma | Locally Advanced | Smoothened Inhibitor | Smoothened Inhibitor | Targeted Therapy | 77 |
| 2015-09-30 | NIVOLUMAB | Melanoma | Accelerated Approval of OPDIVO with YERVOY in BRAF Wild Type Unresectable or Metastatic Melanoma | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 78 |
| 2015-10-27 | TALIMOGENE LAHERPAREPVEC | Melanoma | Unresectable Cutaneous, Subcutaneous, and Nodal Recurrent Melanoma | Locally Advanced | Oncolytic Virus | Oncolytic Virus | Immunotherapy | 79 |
| 2015-10-28 | IPILIMUMAB | Melanoma | Adjuvant Melanoma | Adjuvant | CTLA-4 Ab | CTLA-4 Targeted Antibody | Immunotherapy | 80 |
| 2015-11-10 | COBIMETINIB FUMARATE | Melanoma | Unresectable or Metastatic Melanoma with a BRAF-Mutated Melanoma In Combination with Vemurafenib | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 81 |
| 2015-11-19 | INGENOL MEBUTATE | AK | Describing response to a repeat course | Other | Cytotoxic Agent | Cytotoxic Agent | Cytotoxic Agent | 82 |
| 2015-11-20 | TRAMETINIB DIMETHYL SULFOXIDE | Melanoma | Unresectable or Metastatic Melanoma with a BRAF-Mutated Melanoma In Combination with Dabrafenib | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 83 |
| 2015-11-20 | DABRAFENIB MESYLATE | Melanoma | Regular approval for Dab/Tram in advanced BRAF mutant melanoma | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 84 |
| 2015-11-23 | NIVOLUMAB | Melanoma | Regular Approval for Unresectable or Metastatic BRAF-Wild Type Melanoma | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 85 |
| 2015-12-18 | PEMBROLIZUMAB | Melanoma | Unresectable or Metastatic Melanoma | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 86 |
| 2015-12-18 | PEMBROLIZUMAB | Melanoma | Removing language limiting the indication to disease progression following ipilimumab | Other | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 87 |
| 2016-01-23 | NIVOLUMAB | Melanoma | Accelerated Approved for BRAF Mutant Unresectable or Metastatic Melanoma in the First Line Setting | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 88 |
| 2016-01-23 | NIVOLUMAB | Melanoma | Accelerated approval of Ipi/Nivo Expansion for BRAF mutant as well as BRAF WT | Other | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 89 |
| 2016-05-10 | AMINOLEVULINIC ACID HYDROCHLORIDE | AK | Actinic keratoses of mild-to-moderate severity on the face and scalp | Other | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 90 |
| 2016-09-13 | NIVOLUMAB | Melanoma | Flat Dosing for Melanoma, NSCLC and RCC | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 91 |
| 2016-09-13 | NIVOLUMAB | Melanoma | Flat Dosing for Melanoma, NSCLC and RCC | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 92 |
| 2017-03-23 | AVELUMAB | MCC | Metastatic Merkel Cell Carcinoma | Metastatic | PD-L1 Ab | PD-L1 Targeted Antibody | Immunotherapy | 93 |
| 2017-05-17 | PEMBROLIZUMAB | Melanoma | Flat Dosing of 200 mg q3 weeks in melanoma | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 94 |
| 2017-05-23 | PEMBROLIZUMAB | Agnostic | Unresectable or Metastatic, Microsatellite Instability-High or Mismatch Repair Deficient Solid Tumors | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 95 |
| 2017-07-21 | IPILIMUMAB | Melanoma | Unresectable or Metastatic Melanoma - patients 12 years and older | Locally Advanced or Metastatic | CTLA-4 Ab | CTLA-4 Targeted Antibody | Immunotherapy | 96 |
| 2017-11-09 | BRENTUXIMAB VEDOTIN | CTCL | CD30+ MF and pcALCL in the second line | Refractory | CD30 Ab | CD30 Targeted Antibody | Targeted Therapy | 97 |
| 2017-12-20 | NIVOLUMAB | Melanoma | Regular Approval for Adjuvant Melanoma | Adjuvant | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 98 |
| 2018-01-09 | NIVOLUMAB | Melanoma | Updates infusion from 60 to 30 minutes | Other | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 99 |
| 2018-01-26 | COBIMETINIB FUMARATE | Melanoma | Updates label to include OS data | Other | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 100 |
| 2018-03-05 | NIVOLUMAB | Melanoma | Every 4 Week Dosing for Advanced Melanoma | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 101 |
| 2018-03-05 | NIVOLUMAB | Melanoma | Every 4 week dosing in Adjuvant Melanoma | Adjuvant | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 102 |
| 2018-03-05 | NIVOLUMAB | Melanoma | Updates infusion from 60 to 30 minutes in adjuvant Melanoma | Adjuvant | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 103 |
| 2018-03-06 | AMINOLEVULINIC ACID HYDROCHLORIDE | AK | Minimally to moderately thick Actinic Keratosis of the upper extremities | Other | Phototoxic | Phototoxic Agent | Cytotoxic Agent | 104 |
| 2018-04-30 | TRAMETINIB DIMETHYL SULFOXIDE | Melanoma | Adjuvant BRAF Mutant Melanoma In Combination with Dabrafenib | Adjuvant | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 105 |
| 2018-04-30 | DABRAFENIB MESYLATE | Melanoma | Adjuvant BRAF Mutant Melanoma In Combination with Trametinib | Adjuvant | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 106 |
| 2018-06-27 | ENCORAFENIB | Melanoma | Regular Approval for use in combination with binimetinib for advanced BRAF mutant melanoma | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 107 |
| 2018-06-27 | BINIMETINIB | Melanoma | Unresectable or Metastatic BRAF-Mutated Melanoma In Combination with Encorafenib | Locally Advanced or Metastatic | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 108 |
| 2018-08-08 | MOGAMULIZUMAB-KPKC | CTCL | Relapsed/Refractory Mycosis Fungoides or Sezary Syndrome - Second Line | Refractory | CCR4 Ab | CCR4 Targeted Antibody & ADCC Inducer | Immunotherapy | 109 |
| 2018-09-28 | CEMIPLIMAB-RWLC | SCC | Locally Advanced or Metastatic Squamous Cell Carcinoma | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 110 |
| 2018-12-19 | PEMBROLIZUMAB | MCC | Locally Advanced or Metastatic Merkel Cell Carcinoma | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 111 |
| 2019-01-18 | VISMODEGIB | BCC | To allow for treatment interruptions of up to 8 weeks for intolerable AEs | Other | Smoothened Inhibitor | Smoothened Inhibitor | Targeted Therapy | 112 |
| 2019-02-15 | PEMBROLIZUMAB | Melanoma | Adjuvant Melanoma | Adjuvant | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 113 |
| 2019-03-07 | NIVOLUMAB | Melanoma | Conversion to Regular Approval for the treatment of patients with BRAF V600 mutation-positive unresectable or metastatic melanoma | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 114 |
| 2019-03-07 | NIVOLUMAB | Melanoma | Conversion to Regular Approval of OPDIVO, in combination with ipilimumab, for the treatment of patients with unresectable or metastatic melanoma | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 115 |
| 2019-10-06 | TRAMETINIB DIMETHYL SULFOXIDE | Melanoma | Updating results in subjects with brain metastases | Other | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 116 |
| 2019-10-06 | DABRAFENIB MESYLATE | Melanoma | Updates label in regards to brain mets for melanoma | Other | Kinase Inhibitor | Kinase Inhibitor | Targeted Therapy | 117 |
| 2020-04-28 | PEMBROLIZUMAB | Melanoma | Every 6 weeks dosing in Melanoma | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 118 |
| 2020-04-28 | PEMBROLIZUMAB | MCC | Every 6 weeks dosing in Merkel Cell Carcinoma | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 119 |
| 2020-04-28 | PEMBROLIZUMAB | Agnostic | Every 6 weeks dosing in MSI-H tumors | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 120 |
| 2020-05-14 | POMALIDOMIDE | KS | Treatment of adult patients with AIDS-related Kaposi sarcoma | Other | Immunomodulatory | Cereblon Inhibitor | Imid | 121 |
| 2020-05-14 | POMALIDOMIDE | KS | Treatment of Kaposi’s Sarcoma in patients who are HIV-negative | Other | Immunomodulatory | Cereblon Inhibitor | Imid | 122 |
| 2020-06-16 | PEMBROLIZUMAB | Agnostic | Tumor Mutation Burden-High solid tumors | Other | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 123 |
| 2020-06-16 | PEMBROLIZUMAB | Agnostic | Every 6 weeks dosing in TMB-H tumors | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 124 |
| 2020-06-24 | PEMBROLIZUMAB | SCC | r/mCSCC | Locally Advanced or Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 125 |
| 2020-06-24 | PEMBROLIZUMAB | SCC | Every 6 weeks dosing for CSCC | Dosing Adjustment | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 126 |
| 2020-07-30 | ATEZOLIZUMAB | Melanoma | Unresectable or metastatic BRAF-mutated melanoma in combination with cobimetinib and vemurafenib | Locally Advanced or Metastatic | PD-L1 Ab | PD-L1 Targeted Antibody | Immunotherapy | 127 |
| 2020-12-14 | TIRBANIBULIN | AK | Actinic keratosis of the face or scalp | Other | Microtubule Inhibitor | Microtubule Inhibitor | Cytotoxic Agent | 128 |
| 2021-02-09 | CEMIPLIMAB-RWLC | BCC | Locally Advanced Basal Cell Carcinoma | Locally Advanced | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 129 |
| 2021-02-09 | CEMIPLIMAB-RWLC | BCC | Metastatic Basal Cell Carcinoma | Metastatic | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 130 |
| 2021-07-01 | PEMBROLIZUMAB | SCC | laCSCC | Locally Advanced | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 131 |
| 2021-12-03 | PEMBROLIZUMAB | Melanoma | Adjuvant Stage IIB and IIC Melanoma | Adjuvant | PD-1 Ab | PD-1 Targeted Antibody | Immunotherapy | 132 |
| 2022-01-25 | TEBENTAFUSP-TEBN | Melanoma | HLA-A*02:01-positive adult patients with unresectable or metastatic uveal melanoma | Locally Advanced or Metastatic | Bispecific Antibody | Bispecific gp100 peptide-HLA-A*02:01 directed T cell receptor CD3 T cell engager | Immunotherapy | 133 |
| 2022-03-18 | RELATLIMAB-RMBW | Melanoma | Unresectable or Metastatic Melanoma | Locally Advanced or Metastatic | LAG-3 Ab | LAG-3 Targeted Antibody | Immunotherapy | 134 |
response_rate_df() |>
kable() %>%
kable_styling(bootstrap_options = c("striped","hover"),
fixed_thead = T) %>%
scroll_box(height = "400px",
width = "100%")| name | application_number | Indication | Indication_brief | submission_class_code_description | Action_Date | Dz | Endpoint_shortened | Effect_Estimate | N | Response_Rate | lb_95CI | ub_95CI | lb_95CI_proposed | Toolshort | Mechanism_long | Action_Date_jitter | Drug Name (Indication) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interferon Alfa-2a | BLA103145 | Roferon-A is indicated for the treatment of chronic hepatitis C, hairy cell leukemia and AIDS-related Kaposi's sarcoma in patients 18 years of age or older. In addition, it is indicated for chronic phase, Philadelphia chromosome (Ph) positive chronic myelogenous leukemia (CML) patients who are minimally pretreated (within 1 year of diagnosis). | AIDS KS | Efficacy | 1988-11-21 | KS | Response Rate | NA | 350 | 25.5 | 15.1 | 27.9 | NA | NA | Cytokine | 1988-08-21 | Interferon Alfa-2a (AIDS KS) |
| Interferon Alfa-2b | BLA103132 | INTRON A is indicated for the treatment of selected patients 18 years of age or older with AIDS-Related Kaposi's Sarcoma | AIDS KS | Efficacy | 1988-11-21 | KS | Response Rate | NA | 72 | 29.2 | 19.0 | 41.1 | NA | NA | Cytokine | 1988-11-21 | Interferon Alfa-2b (AIDS KS) |
| Interferon Alfa-2b | BLA103132 | INTRON A is indicated for the treatment of selected patients 18 years of age or older with AIDS-Related Kaposi's Sarcoma | AIDS KS | Efficacy | 1988-11-21 | KS | Response Rate | NA | 23 | 30.4 | 13.2 | 52.9 | NA | NA | Cytokine | 1989-02-21 | Interferon Alfa-2b (AIDS KS) |
| Doxorubicin | NDA050718 | The treatment of AIDS-related Kaposi’s sarcoma in patients after failure or prior systemic chemotherapy or intolerance to such therapy. has progressed on prior combination chemotherapy or in patients who are intolerant to such therapy | AIDS KS (AA) | Type 3 - New Dosage Form | 1995-11-17 | KS | Response Rate | NA | 34 | 27.0 | 12.9 | 44.4 | NA | ACTG | Topoisomerase Inhibitor | 1995-11-17 | Doxorubicin (AIDS KS (AA)) |
| Doxorubicin | NDA050718 | The treatment of AIDS-related Kaposi’s sarcoma in patients after failure or prior systemic chemotherapy or intolerance to such therapy. has progressed on prior combination chemotherapy or in patients who are intolerant to such therapy | AIDS KS (AA) | Type 3 - New Dosage Form | 1995-11-17 | KS | Response Rate | NA | 42 | 48.0 | 32.0 | 63.6 | NA | ACTG | Topoisomerase Inhibitor | 1996-02-17 | Doxorubicin (AIDS KS (AA)) |
| Paclitaxel | NULL | Second-line therapy in patients with AIDS-related Kaposi's sarcoma | AIDS KS | Efficacy | 1997-08-04 | KS | Response Rate | NA | 59 | 59.0 | 45.7 | 71.9 | NA | ACTG | Microtubule Stabilizer | 1997-08-04 | Paclitaxel (AIDS KS) |
| Aldesleukin | BLA103293 | Proleukin is indicated for the treatment of adults with metastatic melanoma | mMelanoma | Efficacy | 1998-01-09 | Melanoma | Objective Response Rate | NA | 270 | 15.9 | 11.8 | 20.8 | NA | 2D Measurement | Cytokine | 1998-01-09 | Aldesleukin (mMelanoma) |
| Methoxsalen | NDA020969 | UVADEX® (methoxsalen) Sterile Solution is indicated for extracorporeal administration with the THERAKOS® UVAR XTS® or THERAKOS® CELLEX® Photopheresis System in the palliative treatment of the skin manifestations of Cutaneous T-Cell Lymphoma (CTCL) that is unresponsive to other forms of treatment | rCTCL | Type 3 - New Dosage Form | 1999-02-25 | CTCL | Response Rate | 12 | 51 | 33.3 | 20.8 | 47.9 | 25 | Composite | Phototoxic Agent | 1999-02-25 | Methoxsalen (rCTCL) |
| Bexarotene | NDA021055 | Targretin® (bexarotene) capsules are indicated for the treatment of cutaneous manifestations of cutaneous T-cell lymphoma in patients who are refractory to at least one prior systemic therapy. | rCTCL | Type 1 - New Molecular Entity | 1999-12-29 | CTCL | Response Rate | 27 | 62 | 32.2 | 20.9 | 45.3 | 5 | Composite | Retinoid | 1999-12-29 | Bexarotene (rCTCL) |
| Bexarotene Gel | NDA021056 | Targretin® (bexarotene) gel 1% is indicated for the topical treatment of cutaneous lesions in patients with CTCL (Stage IA and IB) who have refractory or persistent disease after other therapies or who have not tolerated other therapies. | rCTCL | Type 3 - New Dosage Form | 2000-06-28 | CTCL | Response Rate | 21 | 50 | 26.0 | 14.6 | 40.3 | 5 | Composite | Retinoid | 2000-06-28 | Bexarotene Gel (rCTCL) |
| Vorinostat | NDA021991 | ZOLINZA is a histone deacetylase (HDAC) inhibitor indicated for: Treatment of cutaneous manifestations in patients with cutaneous Tcell lymphoma (CTCL) who have progressive, persistent or recurrent disease on or following two systemic therapies | rCTCL | Type 1 - New Molecular Entity | 2006-10-06 | CTCL | Response Rate | 24.7 | 74 | 29.7 | 19.7 | 41.5 | 5 | mSWAT | HDAC Inhibitor | 2006-07-06 | Vorinostat (rCTCL) |
| Vorinostat | NDA021991 | ZOLINZA is a histone deacetylase (HDAC) inhibitor indicated for: Treatment of cutaneous manifestations in patients with cutaneous Tcell lymphoma (CTCL) who have progressive, persistent or recurrent disease on or following two systemic therapies | rCTCL | Type 1 - New Molecular Entity | 2006-10-06 | CTCL | Response Rate | 24.7 | 33 | 24.2 | 11.1 | 42.3 | 5 | mSWAT | HDAC Inhibitor | 2006-10-06 | Vorinostat (rCTCL) |
| Imatinib | NDA021588 | GLEEVEC is indicated for adult patients with unresectable, recurrent and/or metastatic dermatofibrosarcoma protuberans (DFSP) | u/mDFSP | Efficacy | 2006-10-19 | DFSP | Overall Response Rate | NA | 18 | 83.3 | 58.6 | 96.4 | NA | NA | Kinase Inhibitor | 2007-02-01 | Imatinib (u/mDFSP) |
| Doxorubicin | NDA050718 | DOXIL is an anthracycline topoisomerase inhibitor indicated for AIDS-related Kaposi’s Sarcoma after failure of prior systemic chemotherapy or intolerance to such therapy | AIDS KS | Efficacy | 2008-06-10 | KS | Overall Response Rate | NA | 17 | 41.2 | 18.4 | 67.1 | NA | NA | Topoisomerase Inhibitor | 2008-06-10 | Doxorubicin (AIDS KS) |
| Doxorubicin | NDA050718 | DOXIL is an anthracycline topoisomerase inhibitor indicated for AIDS-related Kaposi’s Sarcoma after failure of prior systemic chemotherapy or intolerance to such therapy | AIDS KS | Efficacy | 2008-06-10 | KS | Overall Response Rate | NA | 11 | 36.4 | 10.9 | 69.2 | NA | NA | Topoisomerase Inhibitor | 2008-09-10 | Doxorubicin (AIDS KS) |
| Romidepsin | NDA022393 | ISTODAX is a histone deacetylase (HDAC) inhibitor indicated for: Treatment of cutaneous T-cell lymphoma (CTCL) in patients who have received at least one prior systemic therapy | rCTCL | Type 1 - New Molecular Entity | 2009-11-05 | CTCL | Objective Response Rate | 19 | 96 | 34.0 | 25.0 | 45.0 | 15 | Composite | HDAC Inhibitor | 2009-08-05 | Romidepsin (rCTCL) |
| Romidepsin | NDA022393 | ISTODAX is a histone deacetylase (HDAC) inhibitor indicated for: Treatment of cutaneous T-cell lymphoma (CTCL) in patients who have received at least one prior systemic therapy | rCTCL | Type 1 - New Molecular Entity | 2009-11-05 | CTCL | Objective Response Rate | 19 | 71 | 35.0 | 25.0 | 49.0 | 15 | Composite | HDAC Inhibitor | 2009-11-05 | Romidepsin (rCTCL) |
| Vismodegib | NDA203388 | ERIVEDGE™ (vismodegib) capsule is a hedgehog pathway inhibitor indicated for the treatment of adults with metastatic basal cell carcinoma, or with locally advanced basal cell carcinoma that has recurred following surgery or who are not candidates for surgery, and who are not candidates for radiation | mBCC | Type 1 - New Molecular Entity | 2012-01-30 | BCC | Objective Response Rate | 20.3 | 22.9 | 33 | 30.3 | 15.6 | 48.2 | 10 | mRECIST | Smoothened Inhibitor | 2012-04-30 | Vismodegib (mBCC) |
| Vismodegib | NDA203388 | ERIVEDGE™ (vismodegib) capsule is a hedgehog pathway inhibitor indicated for the treatment of adults with metastatic basal cell carcinoma, or with locally advanced basal cell carcinoma that has recurred following surgery or who are not candidates for surgery, and who are not candidates for radiation | laBCC | Type 1 - New Molecular Entity | 2012-01-30 | BCC | Objective Response Rate | 20.3 | 22.9 | 63 | 42.9 | 30.5 | 56.0 | 20 | mRECIST | Smoothened Inhibitor | 2012-01-30 | Vismodegib (laBCC) |
| Pembrolizumab | BLA125514 | KEYTRUDA is a human programmed death receptor-1 (PD-1)-blocking antibody indicated for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor. | u/mMelanoma | Type 1 - New Molecular Entity | 2014-09-04 | Melanoma | Overall Response Rate | 13.6 | 173 | 23.6 | 15.0 | 34.0 | 10 | mRECIST | PD-1 Targeted Antibody | 2014-09-04 | Pembrolizumab (u/mMelanoma) |
| Nivolumab | BLA125554 | OPDIVO is a human programmed death receptor-1 (PD-1) blocking antibody indicated for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor. | u/mMelanoma | Type 1 - New Molecular Entity | 2014-12-22 | Melanoma | Overall Response Rate | 17 | 120 | 32.0 | 23.0 | 41.0 | 15 | RECIST | PD-1 Targeted Antibody | 2014-12-22 | Nivolumab (u/mMelanoma) |
| Sonidegib | NDA205266 | ODOMZO is a hedgehog pathway inhibitor indicated for the treatment of adult patients with locally advanced basal cell carcinoma (BCC) that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy. | laBCC | Type 1 - New Molecular Entity | 2015-07-24 | BCC | Objective Response Rate | 38 | 66 | 57.5 | 44.7 | 69.7 | 20 | mRECIST | Smoothened Inhibitor | 2015-07-24 | Sonidegib (laBCC) |
| Avelumab | BLA761049 | BAVENCIO is a programmed death ligand-1 (PD-L1) blocking antibody indicated for the treatment of adults and pediatric patients 12 years and older with metastatic Merkel cell carcinoma (MCC) | mMCC | Type 1 - New Molecular Entity | 2017-03-23 | MCC | Overall Response Rate | 13 | 88 | 33.0 | 23.3 | 43.8 | 20 | RECIST | PD-L1 Targeted Antibody | 2017-03-23 | Avelumab (mMCC) |
| Pembrolizumab | BLA125514 | U.S. Food and Drug Administration granted accelerated approval to pembrolizumab (KEYTRUDA, Merck & Co.) for adult and pediatric patients with unresectable or metastatic, microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumors that have progressed following prior treatment and who have no satisfactory alternative treatment options or with MSI-H or dMMR colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan. | MSI-hi | Efficacy | 2017-05-23 | Agnostic | Objective Response Rate & Duration of Response | NA | 149 | 39.6 | 31.7 | 47.9 | NA | mRECIST | PD-1 Targeted Antibody | 2017-05-23 | Pembrolizumab (MSI-hi) |
| Cemiplimab-rwlc | BLA761097 | LIBTAYO is a programmed death receptor-1 (PD-1) blocking antibody indicated for the treatment of patients with metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC who are not candidates for curative surgery or curative radiation | mCSCC | Type 1 - New Molecular Entity | 2018-09-28 | SCC | Overall Response Rate | 31.7 | 23.5 | 75 | 46.7 | 35.1 | 58.6 | 15 | Composite | PD-1 Targeted Antibody | 2018-06-28 | Cemiplimab-rwlc (mCSCC) |
| Cemiplimab-rwlc | BLA761097 | LIBTAYO is a programmed death receptor-1 (PD-1) blocking antibody indicated for the treatment of patients with metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC who are not candidates for curative surgery or curative radiation | laCSCC | Type 1 - New Molecular Entity | 2018-09-28 | SCC | Overall Response Rate | 31.7 | 23.5 | 33 | 48.5 | 30.8 | 66.5 | 25 | Composite | PD-1 Targeted Antibody | 2018-09-28 | Cemiplimab-rwlc (laCSCC) |
| Pembrolizumab | BLA125514 | For the treatment of adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma | la/mMCC | Efficacy | 2018-12-19 | MCC | Overall Response Rate & Duration of Response | NA | 50 | 56.0 | 41.0 | 70.0 | NA | RECIST | PD-1 Targeted Antibody | 2018-12-19 | Pembrolizumab (la/mMCC) |
| Trametinib | NDA204114 | Updating results in subjects with BRAF mutation-positive melanoma that has metastasized to the brain | mMelanoma | Efficacy | 2019-10-06 | Melanoma | Overall Response Rate | NA | 121 | 50.0 | 40.0 | 60.0 | NA | mRECIST | Kinase Inhibitor | 2019-07-06 | Trametinib (mMelanoma) |
| Dabrafenib | NDA202806 | Updates to the CLINICAL STUDIES (14.2) section in the TAFINLAR United States Prescribing Information (USPI) adding the results of Study BRF117277 (Novartis study code CDRB436B2204; “the COMBI-MB study”), a non-randomized, open-label, multi-center, multi-cohort trial of dabrafenib plus trametinib in subjects with BRAF mutation-positive melanoma that has metastasized to the brain | mMelanoma | Efficacy | 2019-10-06 | Melanoma | Overall Response Rate | NA | 121 | 50.0 | 40.0 | 60.0 | NA | mRECIST | Kinase Inhibitor | 2019-10-06 | Dabrafenib (mMelanoma) |
| Pomalidomide | NDA204026 | New indication for the treatment of adult patients with AIDS-related Kaposi sarcoma (KS) after failure of highly active antiretroviral therapy (HAART) | HIV-pos KS | Efficacy | 2020-05-14 | KS | Objective Response Rate | NA | 18 | 67.0 | 41.0 | 86.6 | NA | ACTG | Cereblon Inhibitor | 2020-05-01 | Pomalidomide (HIV-pos KS) |
| Pomalidomide | NDA204026 | Treatment of Kaposi’s Sarcoma in patients who are HIV-negative | HIV-neg KS | Efficacy | 2020-05-14 | KS | Objective Response Rate | NA | 10 | 80.0 | 44.4 | 97.5 | NA | ACTG | Cereblon Inhibitor | 2020-05-14 | Pomalidomide (HIV-neg KS) |
| Pembrolizumab | BLA125514 | This supplement modifies the previous label for the treatment of adult and pediatric patients with unresectable or metastatic tumor mutational burden-high (TMB-H) [≥10 mutations/megabase (mut/Mb)] solid tumors, as determined by an FDA-approved test, that have progressed following prior treatment and who have no satisfactory alternative treatment options | TMB-hi | Efficacy | 2020-06-16 | Agnostic | Objective Response Rate & Duration of Response | NA | 102 | 29.0 | 21.0 | 39.0 | NA | mRECIST | PD-1 Targeted Antibody | 2020-06-16 | Pembrolizumab (TMB-hi) |
| Pembrolizumab | BLA125514 | This Prior Approval supplemental biologics application adds a new indication for KEYTRUDA, for the treatment of patients with recurrent or metastatic cutaneous squamous cell carcinoma (cSCC) that is not curable by surgery or radiation | r/mCSCC | Efficacy | 2020-06-24 | SCC | Objective Response Rate | 34.299999999999997 | 105 | 34.3 | 25.2 | 44.2 | 15 | mRECIST | PD-1 Targeted Antibody | 2020-08-24 | Pembrolizumab (r/mCSCC) |
| Cemiplimab-rwlc | BLA761097 | For the treatment of patients with locally advanced BCC (laBCC) previously treated with a hedgehog pathway inhibitor or for whom a hedgehog pathway inhibitor is not appropriate | laBCC | Efficacy | 2021-02-09 | BCC | Objective Response Rate | NA | 112 | 29.0 | 19.0 | 40.0 | 20 | Composite | PD-1 Targeted Antibody | 2020-11-09 | Cemiplimab-rwlc (laBCC) |
| Cemiplimab-rwlc | BLA761097 | For the treatment of patients with metastatic BCC (mBCC) previously treated with a hedgehog pathway inhibitor or for whom a hedgehog pathway inhibitor is not appropriate | mBCC | Efficacy | 2021-02-09 | BCC | Objective Response Rate | NA | 112 | 21.0 | 8.0 | 41.0 | NA | Composite | PD-1 Targeted Antibody | 2021-02-09 | Cemiplimab-rwlc (mBCC) |
| Pembrolizumab | BLA125514 | A new indication for pembrolizumab for locally advanced cSCC that is not curable by surgery or radiation | laCSCC | Efficacy | 2021-07-01 | SCC | Objective Response Rate | NA | 54 | 50.0 | 36.0 | 64.0 | NA | mRECIST | PD-1 Targeted Antibody | 2021-07-01 | Pembrolizumab (laCSCC) |
If you encounter a clear bug, please file an issue with a minimal reproducible example on GitHub.
skincancerRx is for research purposes only. No clinical decisions should be made with the information obtained from its output.