Should Ipilimumab Be the New “Standard” for Refractory MCC?
PlumX
Altmetric
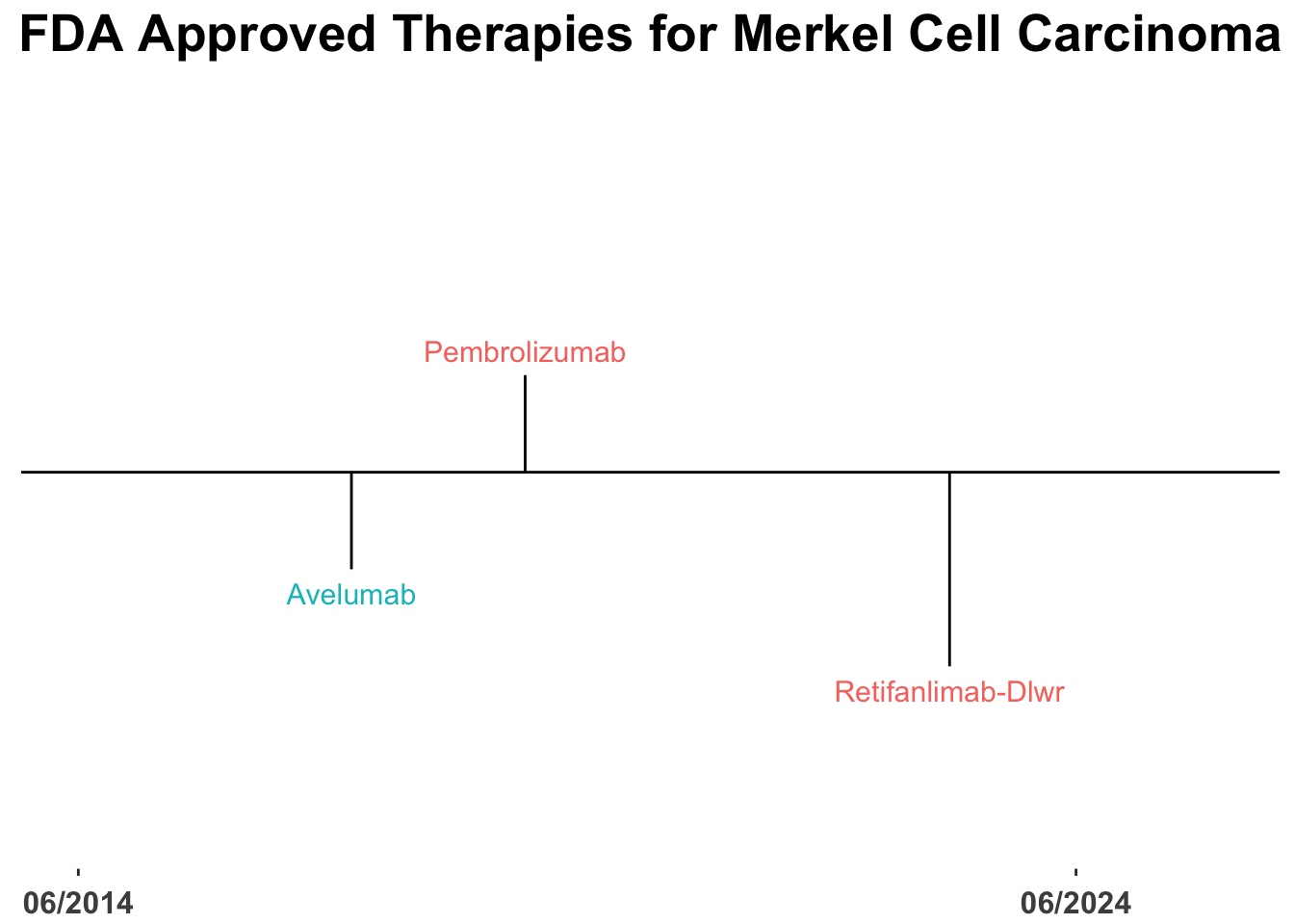
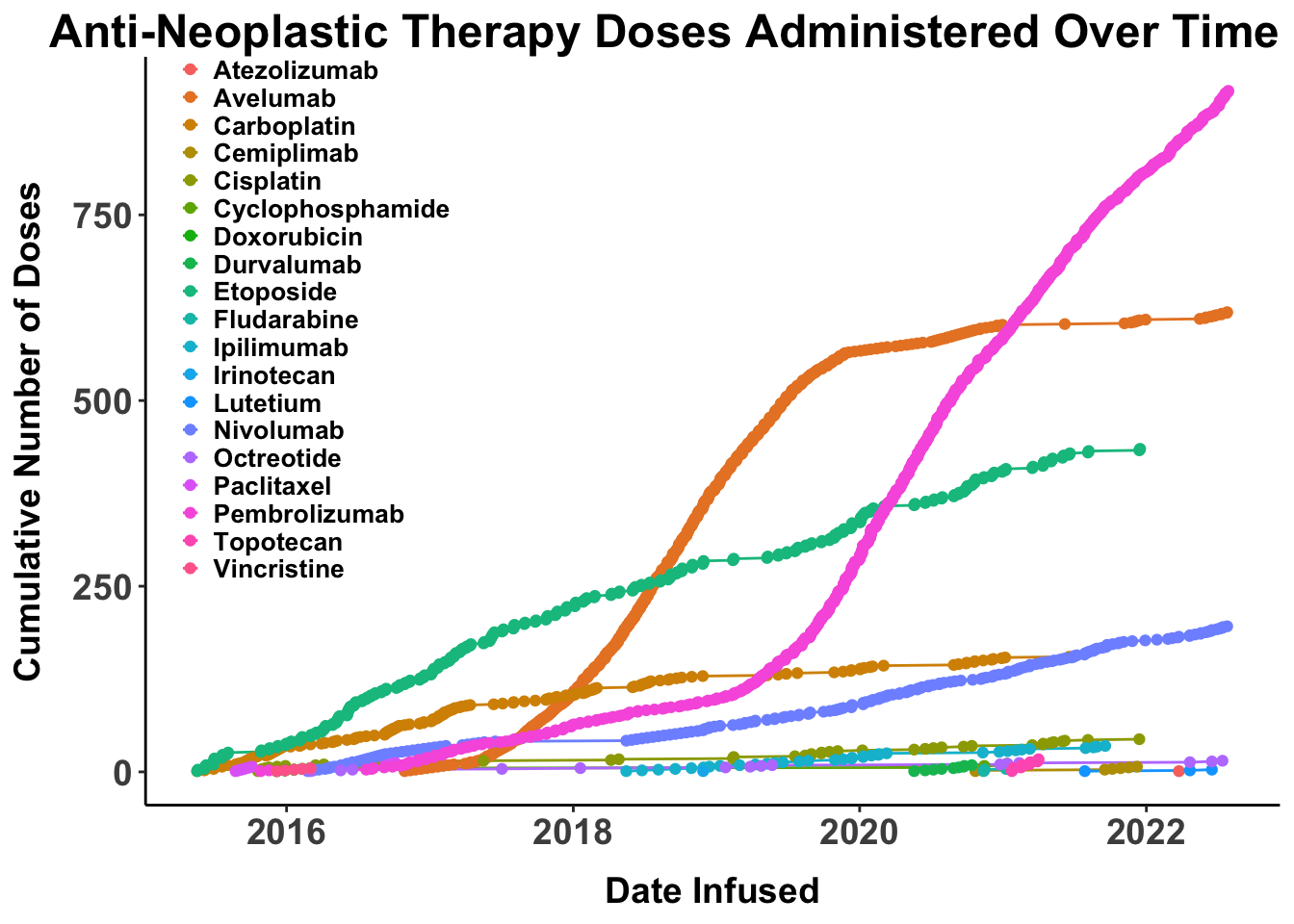
The therapeutic landscape for skin cancer continues to advance rapidly (Figure 1).1 However, a relatively small fraction of US Food and Drug Administration (FDA) approvals in cutaneous oncology are for treatments targeting Merkel cell carcinoma (MCC) (Figure 2). Notably, the introduction of monoclonal antibodies (mAbs) targeting the Programmed Death-1(PD-1)/Programmed Death Ligand-1(PD-L1) pathway has markedly enhanced outcomes for patients with advanced MCC, with more than 50% benefiting from front-line therapies that incorporate these agents (Table 1).2–6 Such regimens have fundamentally transformed the management of advanced disease, as evidenced by the evolving therapeutic approaches at Brigham and Women’s Hospital (BWH)/Dana-Farber Cancer Institute (DFCI) and Massachusetts General Hospital (MGH) in Boston (Figure 3).
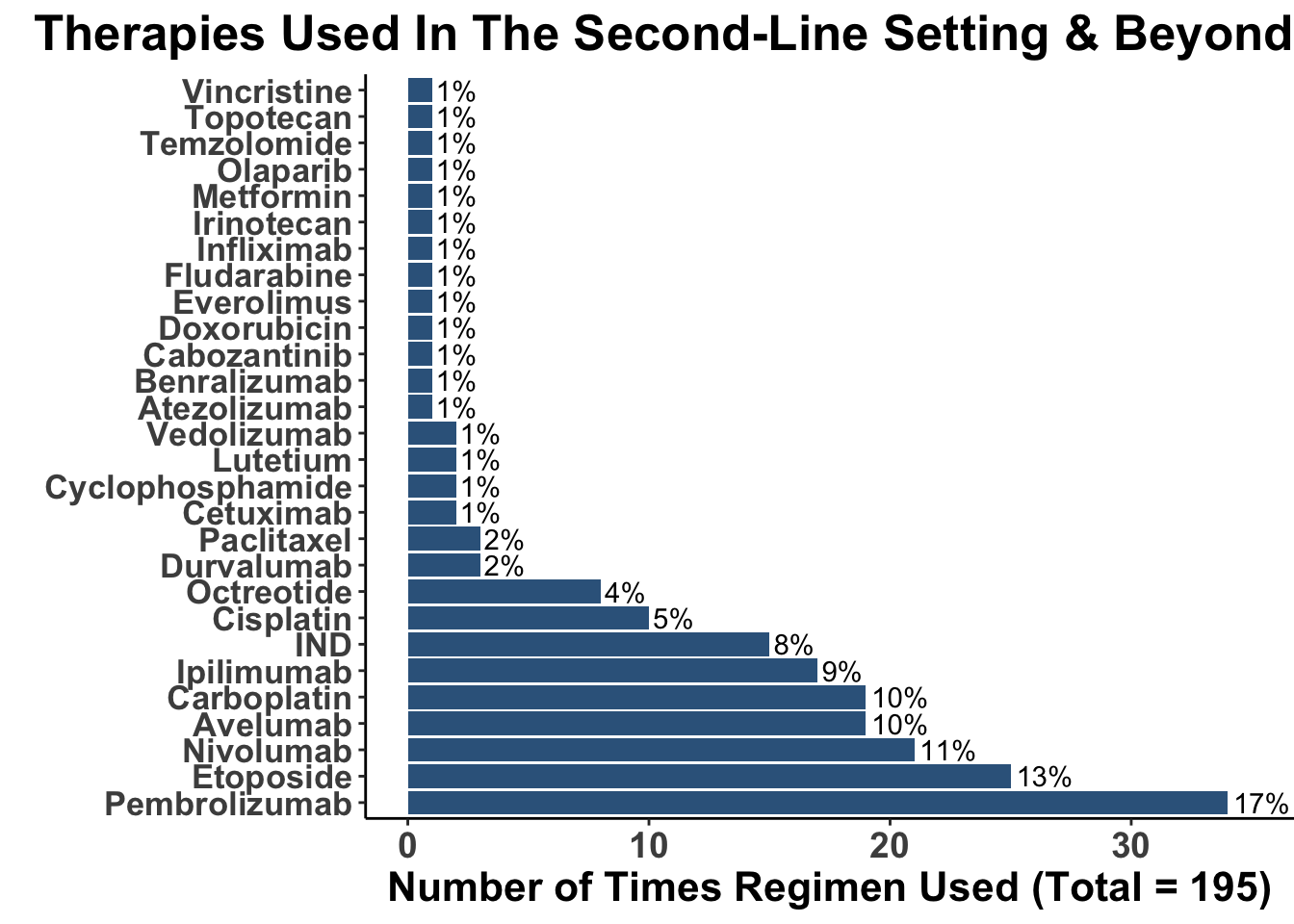
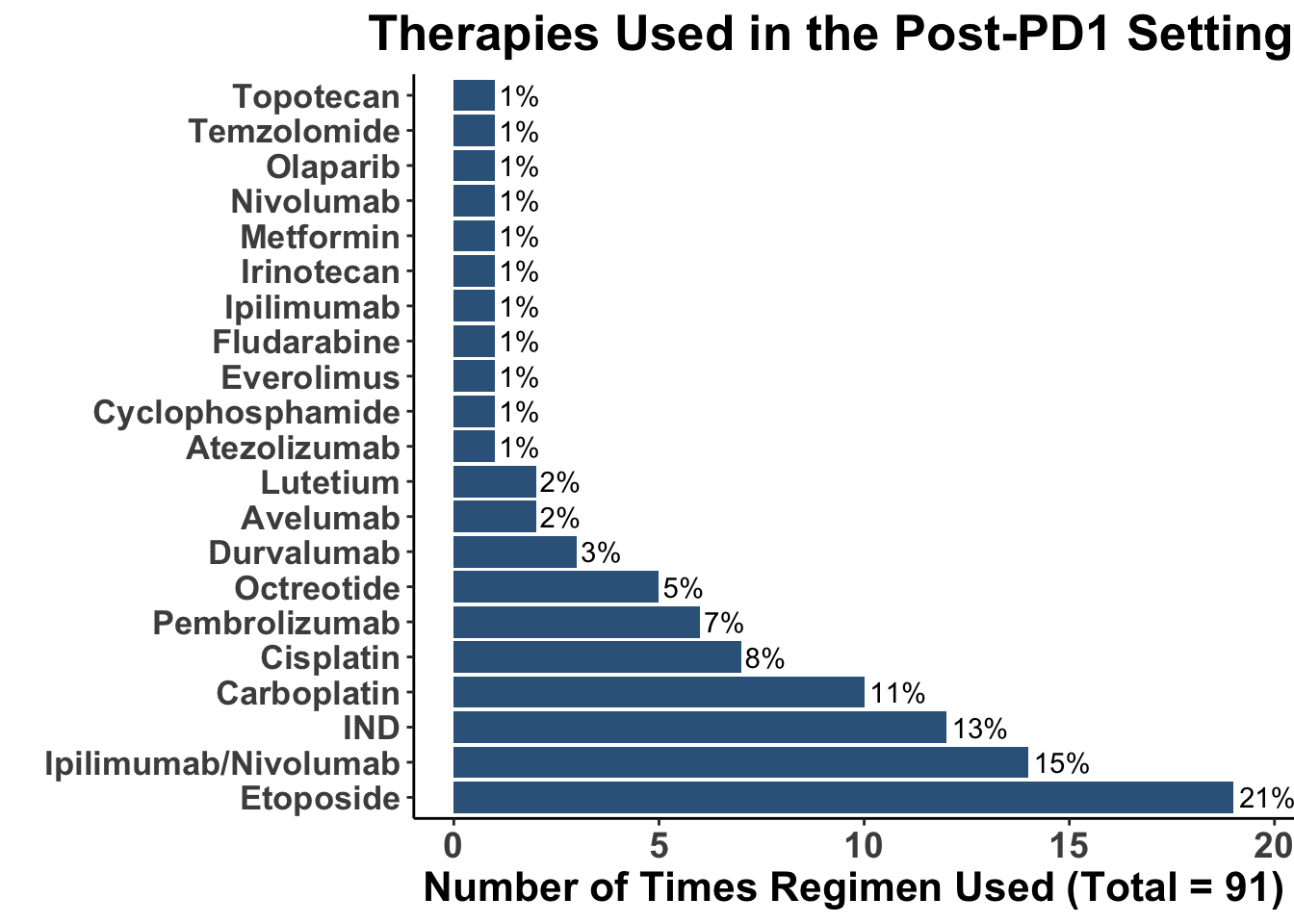
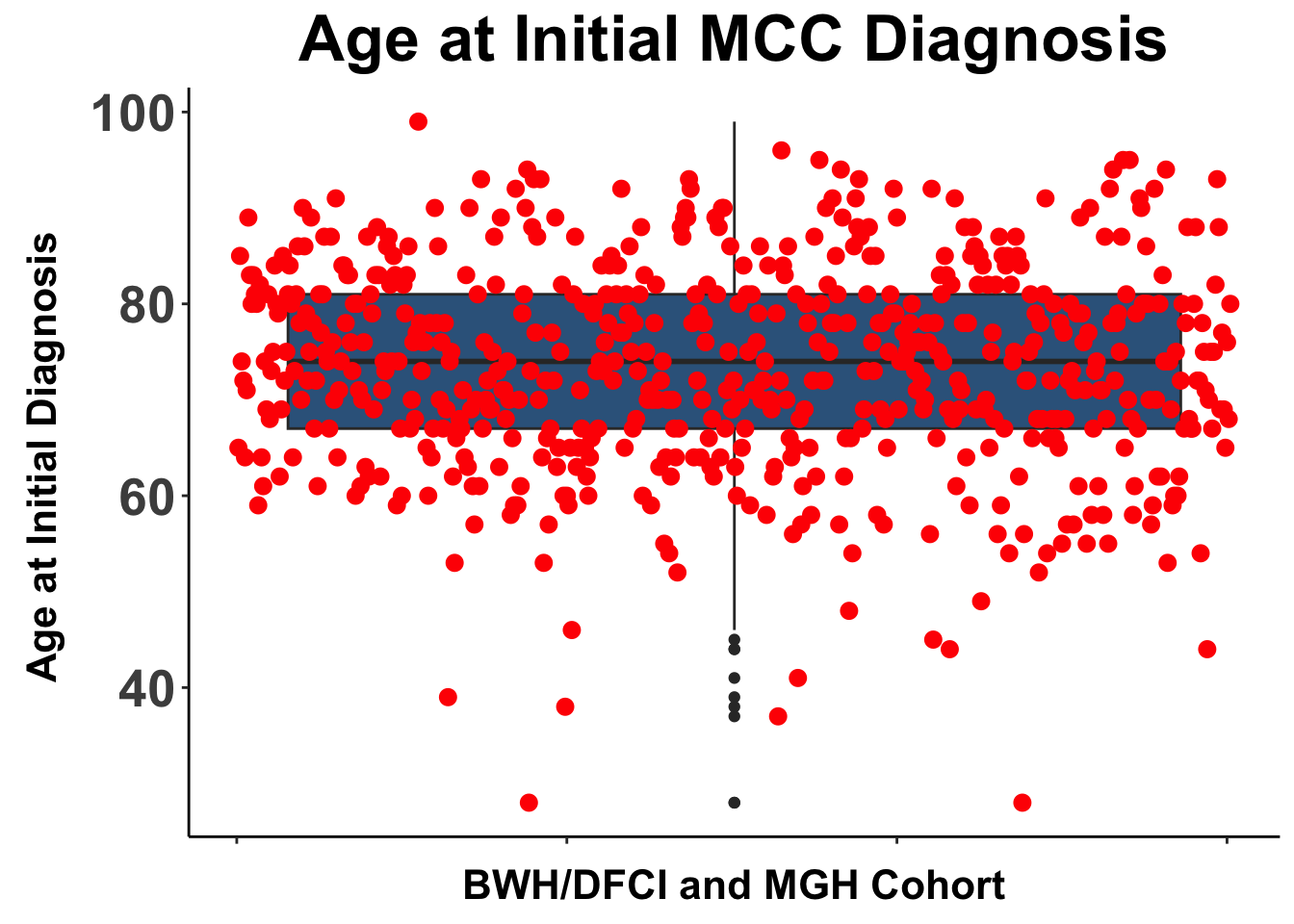
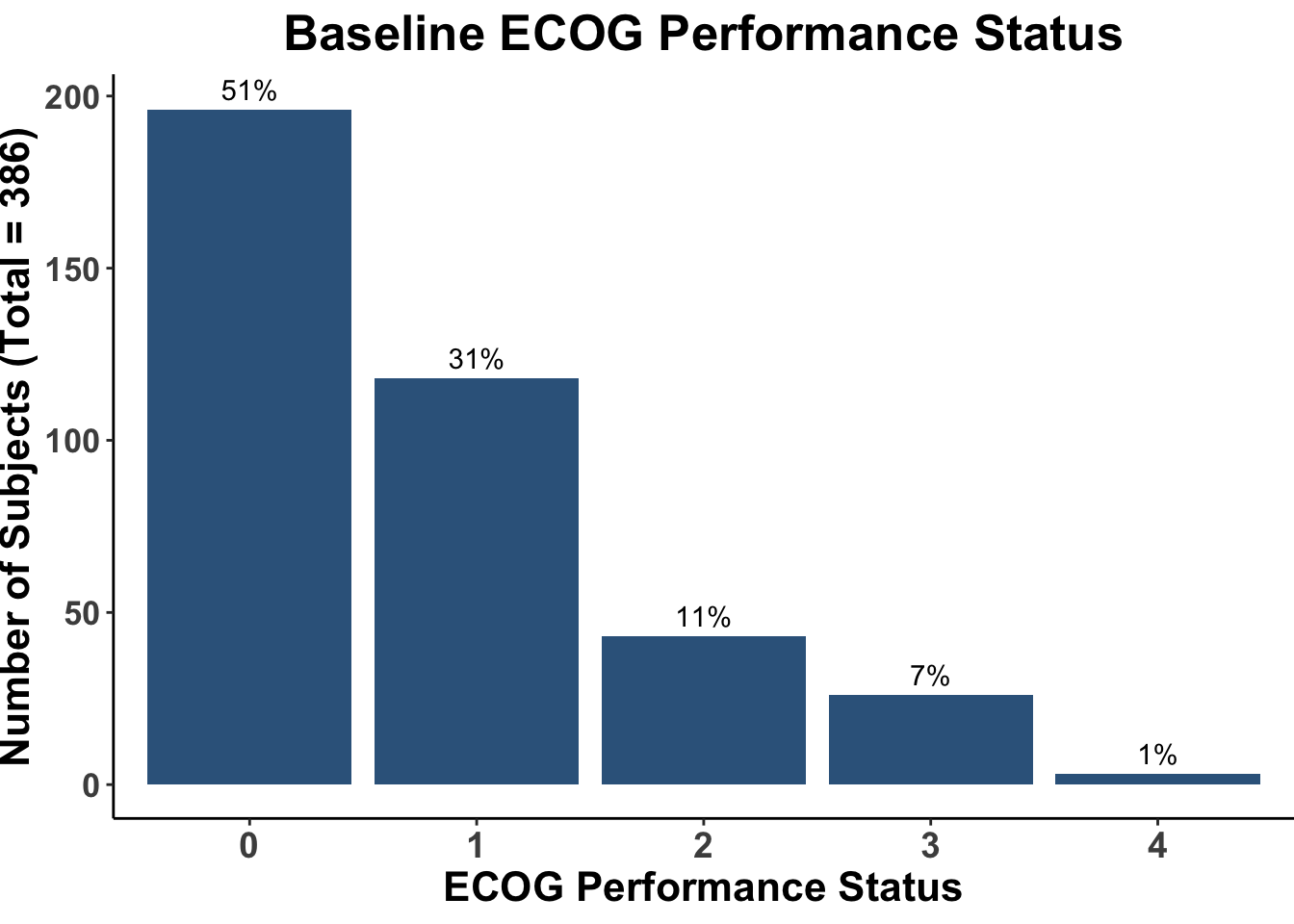
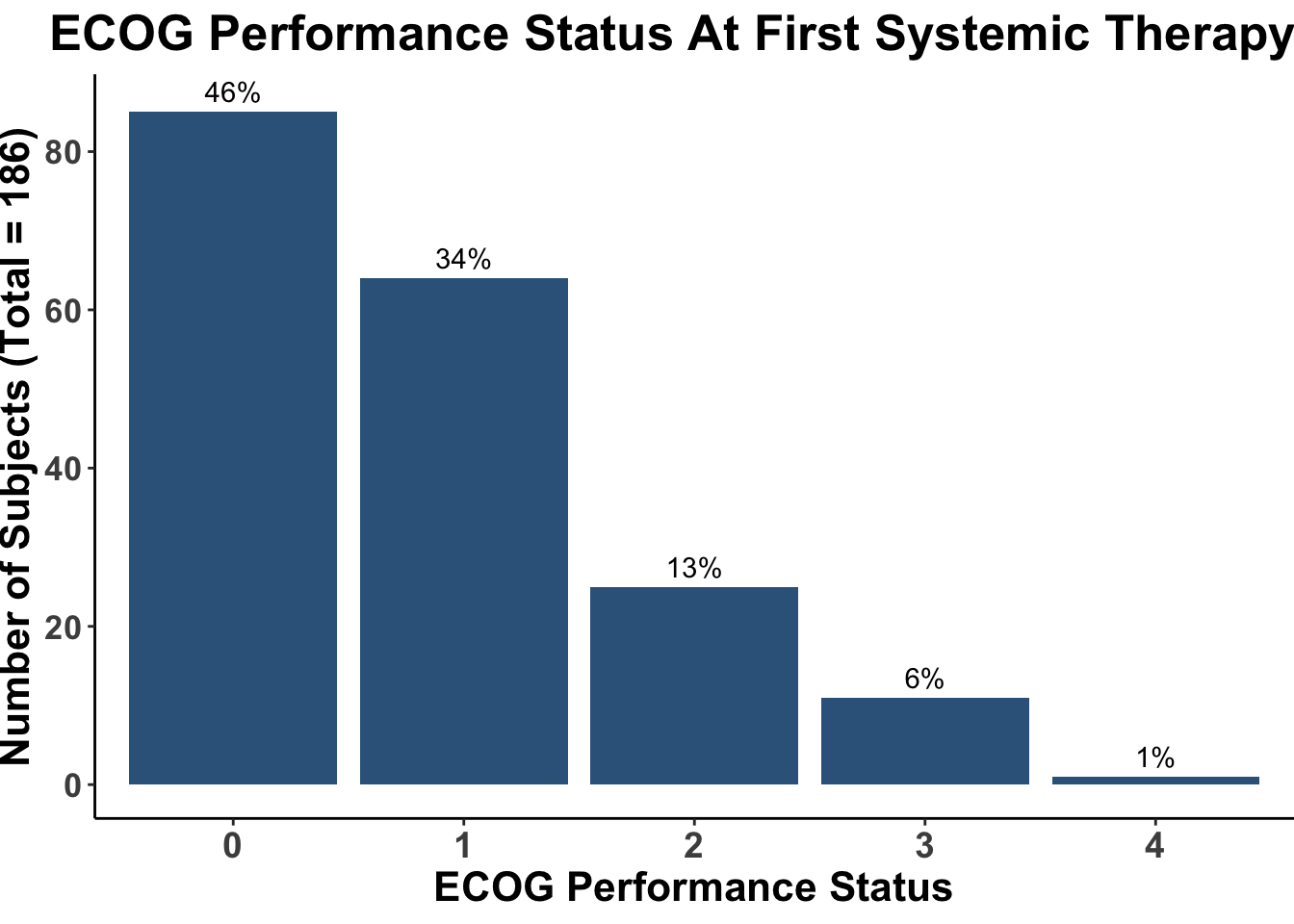
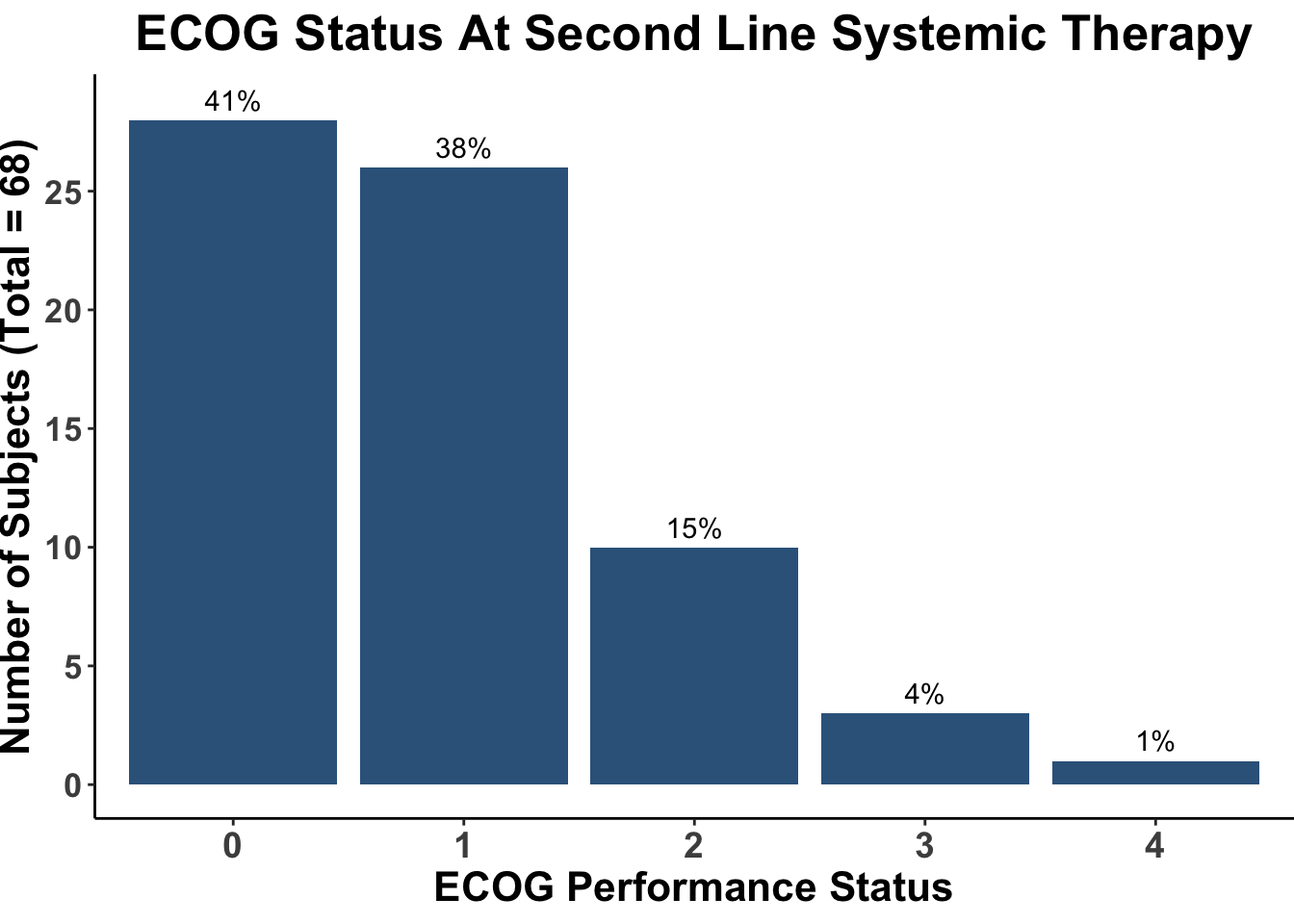
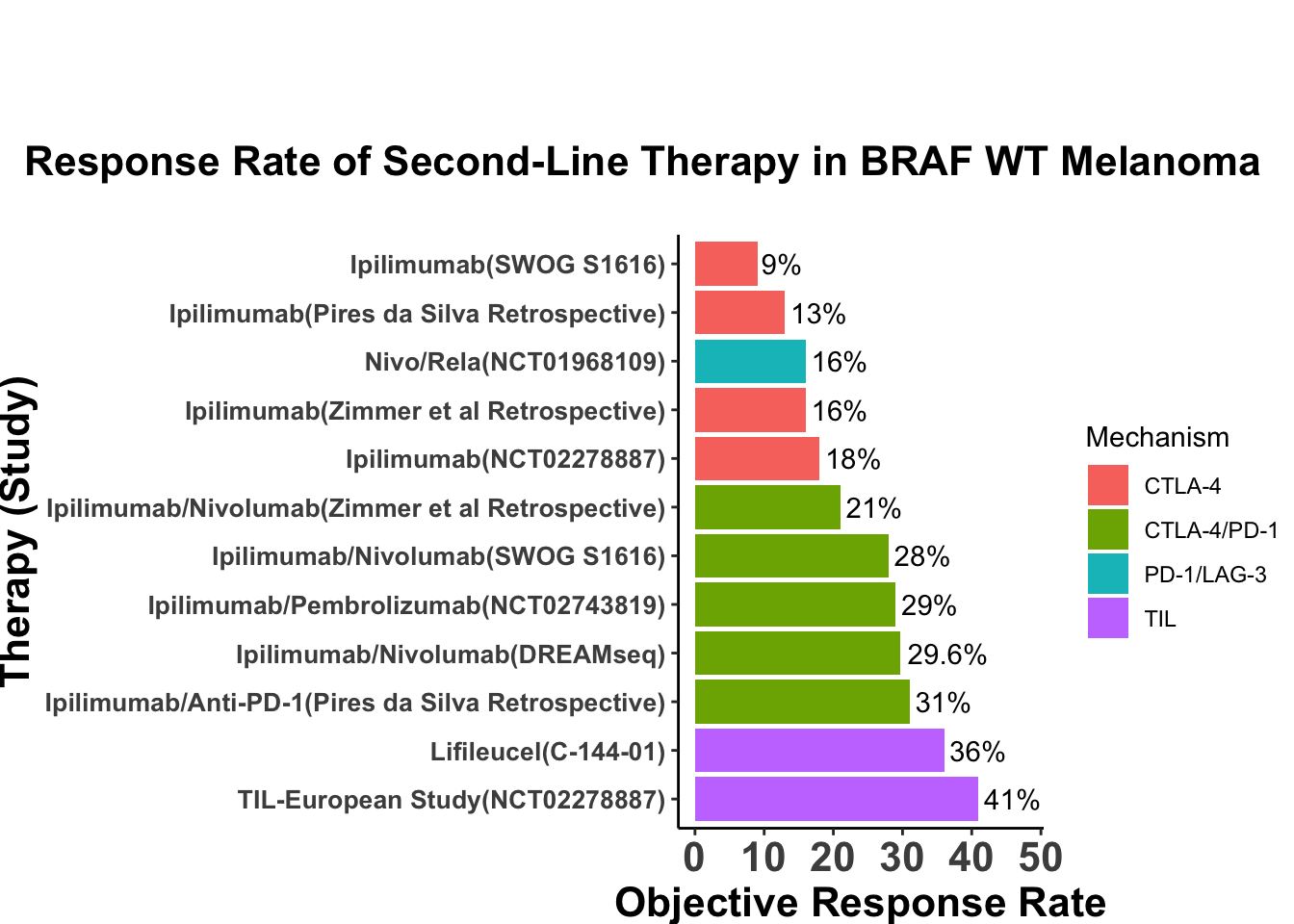
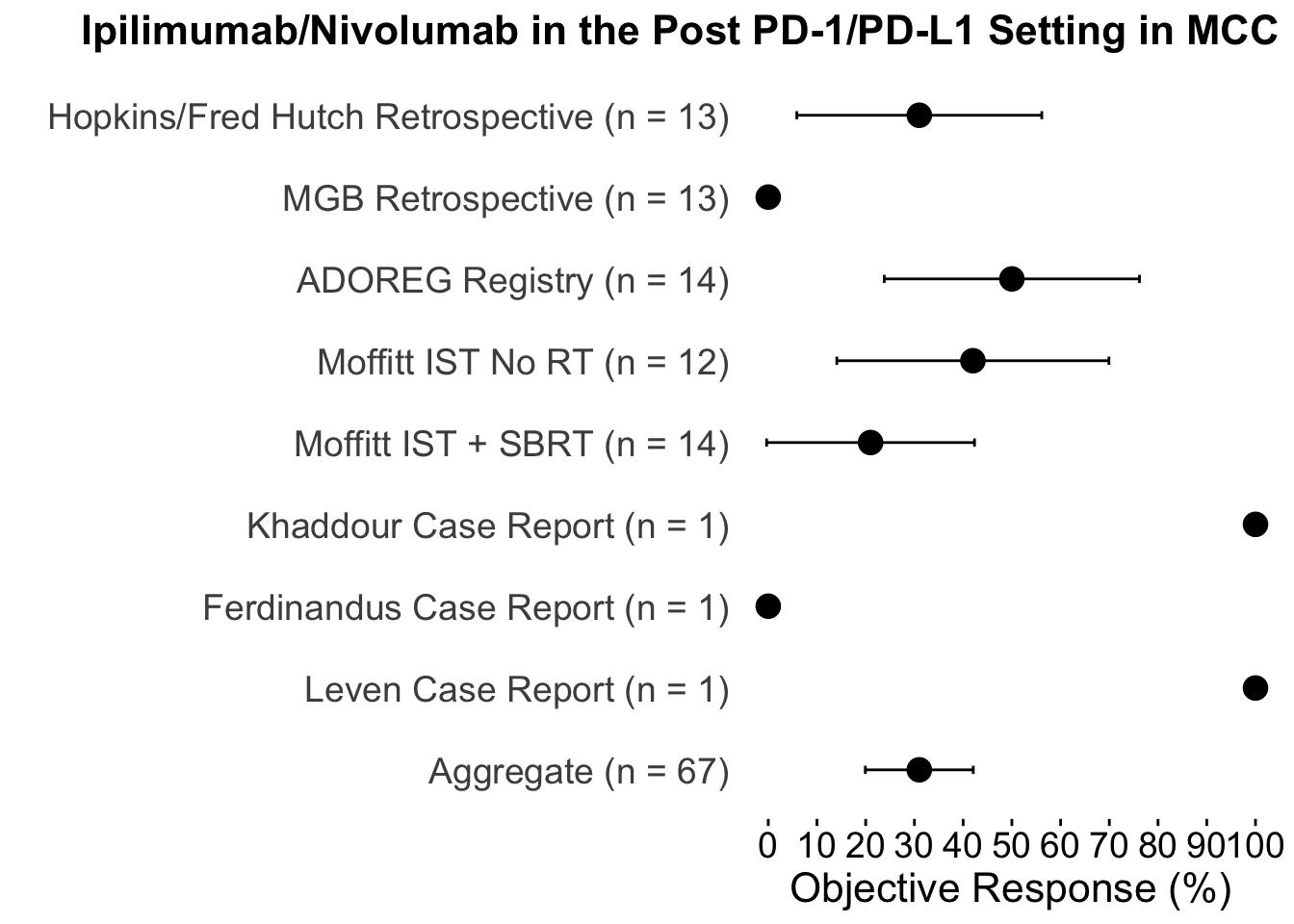
Despite these advances, a significant unmet need persists in the post-anti-PD-1/PD-L1 setting, where no FDA-approved therapies specifically exist for second-line use. Our experience indicates a diverse array of agents employed in this phase (Figures 4 and 5), highlighting the lack of consensus on second-line treatments. Ideally, all patients in this setting would participate in clinical trials. Yet, logistical challenges, such as the rarity of MCC, the advanced age of patients at diagnosis (Figure 6), and their poor performance status at diagnosis and when systemic therapy becomes necessary (Figures 7-9), often preclude trial enrollment. In response, many clinicians resort to anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) therapies, with or without PD-1/PD-L1 inhibitors, in the second-line setting.8 The combination of ipilimumab (an anti-CTLA-4 mAb) with Nivolumab (an anti-PD-1 mAb), approved for advanced melanoma for over a decade, is an option for melanoma patients progressing after first-line PD-1 therapy, achieving objective response rates (ORR) of 21-31% (Figure 10). This data has led clinicians to increasingly consider ipilimumab for patients with PD-1/PD-L1 refractory MCC.
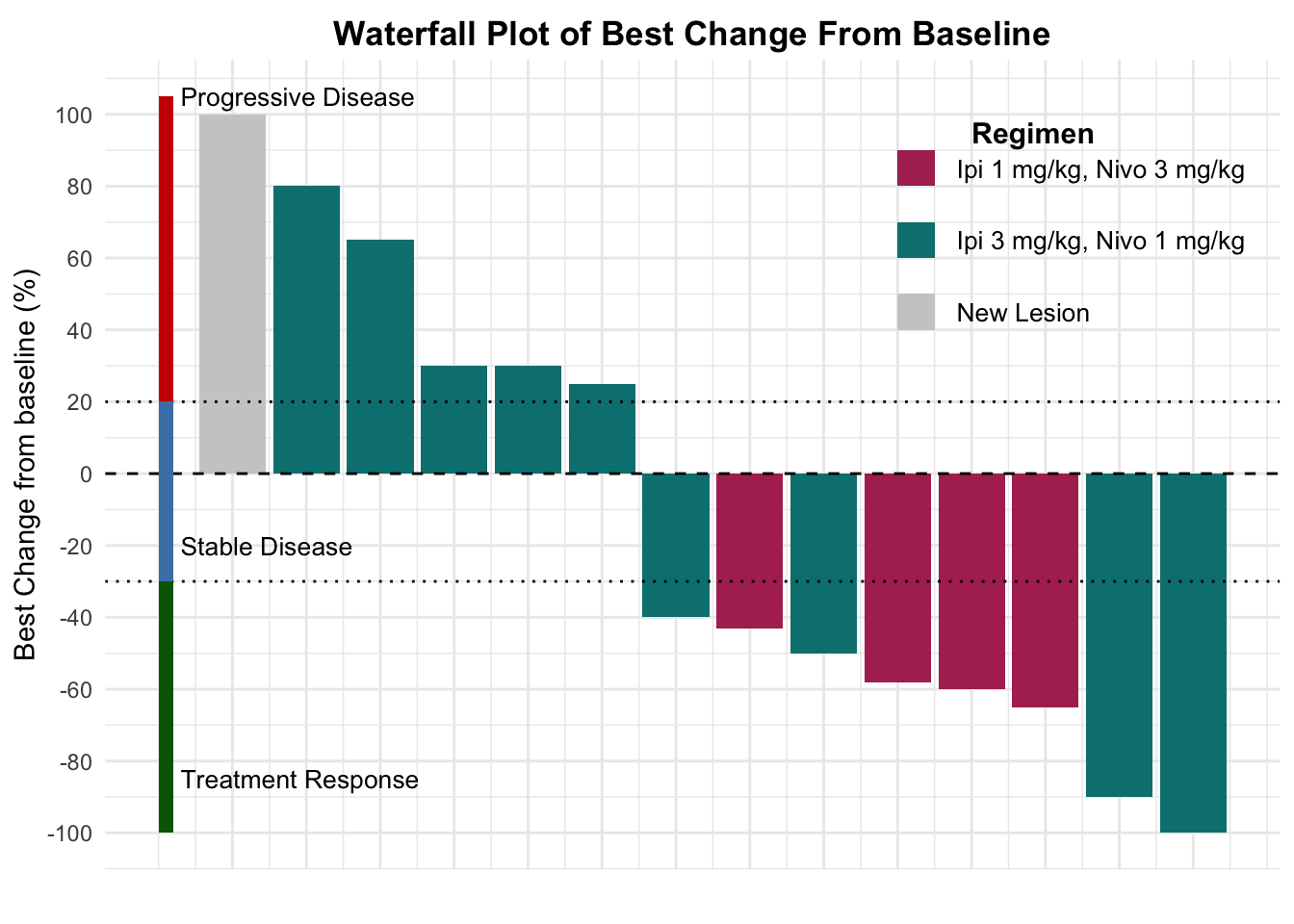
Before the approval of PD-1/PD-L1 inhibitors, ipilimumab demonstrated single-agent efficacy in patients with advanced disease (Table 2).14 Significant developments occurred in 2019, illuminating the potential of combined CTLA-4 and PD-1 blockade in PD-1/PD-L1 refractory MCC. Glutsch et al. described a 60-year-old male with progressive disease on avelumab who achieved a complete response after receiving four doses of ipilimumab (1 mg/kg) and nivolumab (3 mg/kg).15 Later in the year, LoPiccolo et al. documented outcomes for 13 patients at Johns Hopkins and Fred Hutchinson Cancer Center, where a regimen of ipilimumab/nivolumab led to one complete and three partial responses, culminating in an ORR of 31% (Table 3).16
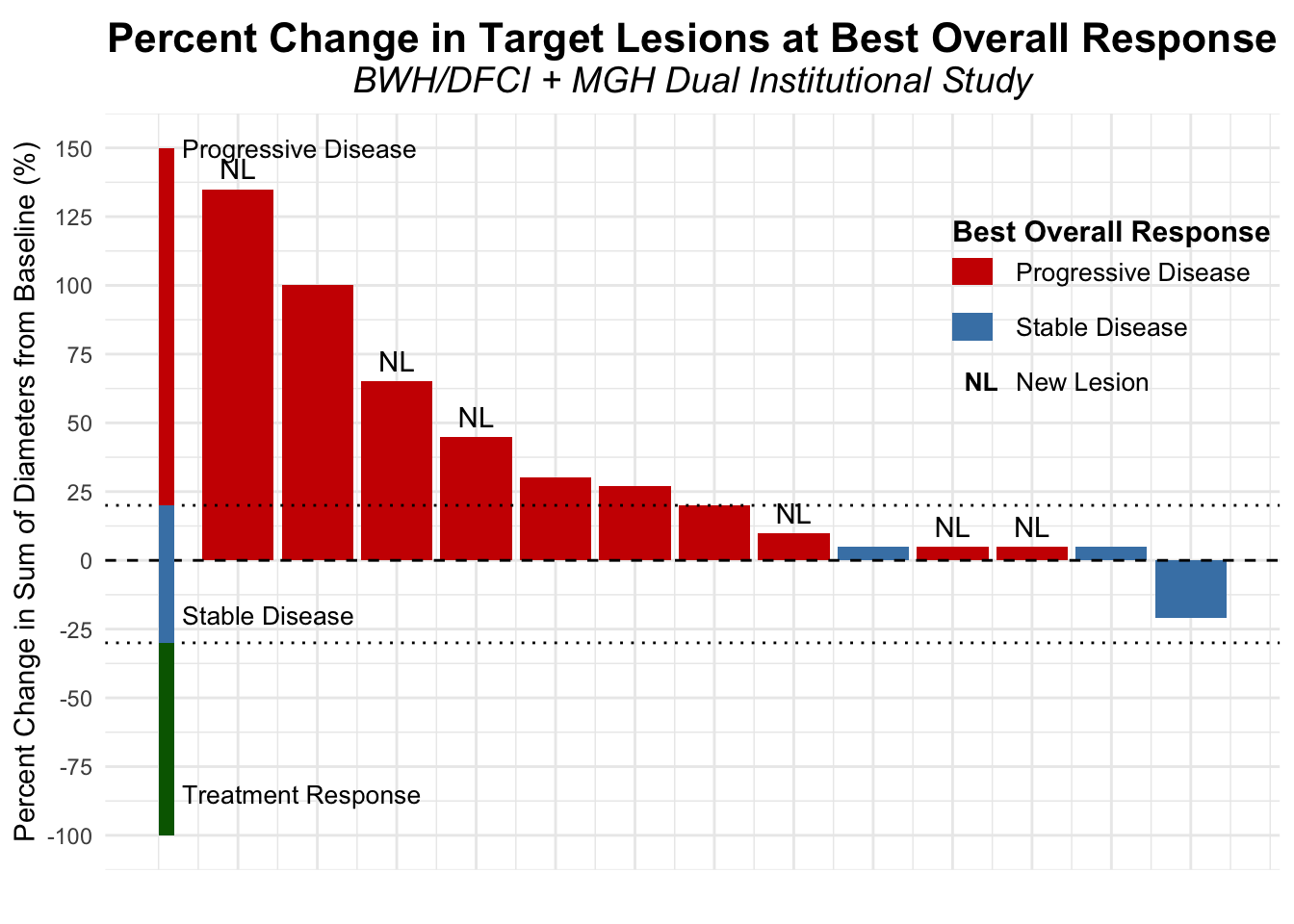
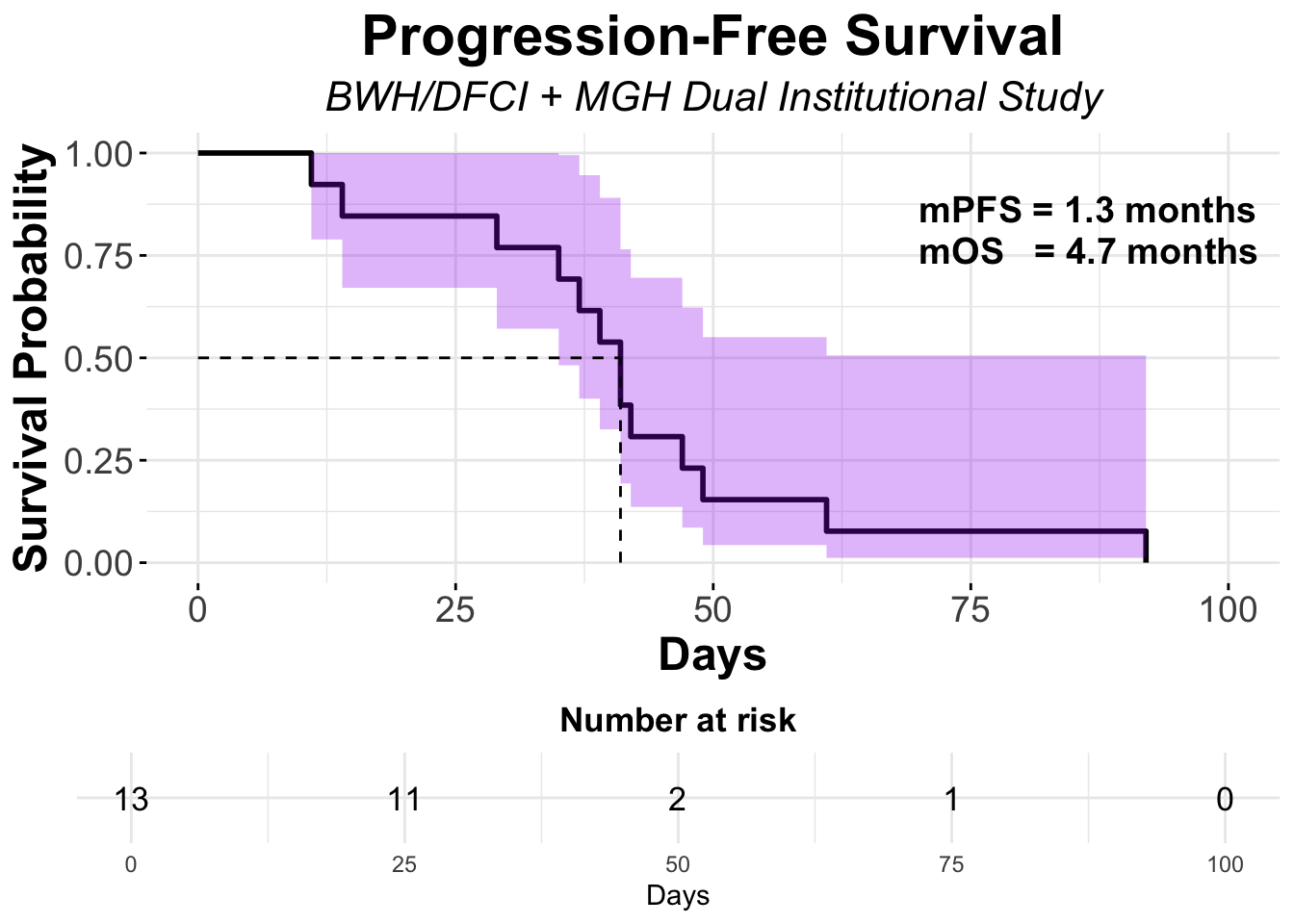
Contrasting these findings, our dual-institutional study at BWH/DFCI and MGH reported no treatment responses among 13 patients treated with the same combination in the post PD-1/PD-L1 setting (Figures 11-12).17 However, the ADOREG multicenter skin cancer registry provided more optimistic results, with Glutsch et al. reporting a 50% response rate in 14 patients following progression on avelumab (Figure 13), and over 60% of these patients were still alive three years after initiating treatment (Table 4).18
In the only prospective clinical trial conducted to date on this subject, Kim et al. studied 26 patients who had progressed following PD-1/PD-L1 therapy. The patients were assigned to receive either ipilimumab plus nivolumab alone, or in combination with stereotactic body radiotherapy (SBRT). The trial reported an ORR of 31% with no significant enhancement from the addition of SBRT (Table 5).19 This response rate of 31% is consistent with the collective body of data, including subsequent case reports, across 67 patients treated with the combination therapy (Table 6 and Figure 14).20–22
It is important to weigh these outcomes against the known toxicity profile of combination anti-CTLA-4/anti-PD-1 therapy. When administered at the FDA-approved dosage for melanoma1, more than 50% of patients experience serious adverse events (SAEs) (Table 7). Conversely, smaller second-line studies in MCC have reported slightly lower CTCAE grade 3-4 adverse event rates of 29-35%, compared to larger registration trials which consistently reported rates exceeding 50% (Table 8). This variation in toxicity rates may be attributed in part to the heterogeneity in dosages used across these studies. Notably, the prospective study by Kim et al. utilized a modified regimen, administering ipilimumab at 1 mg/kg every six weeks along with nivolumab 3 mg/kg every three weeks, which diverges from the original FDA-approved dose for combination anti-CTLA-4/anti-PD-1.
1 The original FDA approval for combination ipilimumab/nivolumab was for the following dosage: ipilimumab 3 mg/kg with Nivolumab 1 mg/kg, every three weeks. Subsequent regimens, including Nivolumab 3 mg/kg with ipilimumab 1 mg/kg every three weeks, have been explored and are potentially better tolerated, as discussed previously previously discussed.
Given these data, how should we interpret the current evidence regarding ipilimumab for refractory MCC? One useful framework is the statutory standards that must be met by products for labeling at the US Food and Drug Administration. US regulatory standards mandate that for a product to receive regular approval, the therapy must demonstrate direct clinical benefits, such as improvements in how a patient feels, functions, or survives.1 Typically, investigators must establish substantial evidence of clinical benefits by demonstrating the isolation of the therapy’s effect, statistical persuasiveness of the endpoint, and external duplication of the results.
Applying these criteria to the body of evidence surrounding anti-CTLA-4/anti-PD-1 therapy for refractory MCC reveals that while the data suggests an effect, it predominantly impacts surrogate endpoints rather than direct clinical benefits. As outlined in the “Guidance for Industry - Expedited Programs for Serious Conditions,” a surrogate endpoint is one “that is reasonably likely to predict clinical benefits or can be measured earlier than irreversible morbidity or mortality and is likely to predict these outcomes”.24 ORR often serves as such a surrogate or intermediate clinical endpoint in oncology trials.
In the context of ipilimumab/nivolumab treatment, evaluating survival metrics such as progression-free survival (PFS) and overall survival (OS) is critical to understanding the therapy’s clinical benefit. However, the available data, derived from single-arm studies, lack appropriate comparator arms, thereby limiting the ability to definitively isolate the treatment’s impact on these time-to-event endpoints. Even the prospective trial by Kim et al., which included two treatment arms, did not differentiate the effects of therapy as both arms featured combinations of ipilimumab/nivolumab.19 In contrast, the nature of malignant tumors, which generally do not regress without active intervention, makes ORR a practical method for assessing treatment effects in the absence of a comparator group. This approach, however, can be complicated by phenomena such as the “Lazarus Effect,” where late responses mimic treatment effects.25 This is particularly relevant when evaluating treatment effects in the second-line and beyond setting.
The statistical persuasiveness of the current data is supported by an aggregate ORR of 31%, aligning with similar findings in melanoma studies and the prospective study from Moffitt/Ohio State University. The lower bound confidence interval of 20% reduces the likelihood that the observed ORR results solely from rare phenomena like delayed responses. While publication bias could potentially skew the aggregate data, as studies reporting negative outcomes are less frequently published, the inclusion of studies reporting zero responders among the seven examined adds robustness to the dataset. Additionally, the overall consistency in ORR across these studies helps mitigate some concern about potential publication bias.
This analysis underscores the need for rigorous comparative studies to further validate the efficacy of ipilimumab/nivolumab in the refractory MCC setting, aligning with FDA standards for clinical benefit. However, the realities of treating a rare disease like MCC often limit the feasibility of conducting such studies. Thus, medical decisions sometimes must be made based on less than ideal data, making it crucial to use the best available evidence to guide treatment choices.
The variability in response rates across different studies, such as those reported by Shalhout et al.17, Glutsch et al.18, and Kim et al.19, may be attributable to differences in patient populations, particularly regarding their baseline health and performance status. Our study observed zero responders, potentially reflecting a cohort with poorer performance status and more advanced disease at the onset of treatment.17 This contrasts with the more promising results from the Glutsch et al.18 and Kim et al.19 studies, where patients might have been healthier or received treatment at an earlier stage of disease progression. These observations suggest the potential benefit of a more proactive approach in managing MCC: evaluating patient response to first-line anti-PD-1 therapy as early as after two doses. If there is no evidence of treatment response, it might be prudent to swiftly transition these patients to second-line ipilimumab/nivolumab therapy. Delaying this evaluation and subsequent treatment shift could lead to further declines in a patient’s performance status, potentially diminishing their ability to benefit from second-line therapies. While this approach is speculative and would require validation through clinical trials, in the absence of such data, it seems reasonable to consider this practice to optimize outcomes for patients with MCC.
Upon careful analysis, the current body of data supports the use of ipilimumab/nivolumab in the post-PD-1/PD-L1 setting for select patients with MCC. This therapy may be particularly suitable for younger patients with good performance status, who are more likely to tolerate and benefit from intensive treatment approaches. However, given the significant heterogeneity in the MCC patient population, it is imperative to consider personalized treatment strategies. For some patients, especially those with advanced age or poor performance status, the potential benefits of extensive cancer-directed therapy may not outweigh the risks associated with high toxicity levels. In such cases, prioritizing quality of life through comfort care might be the most appropriate approach.
Ideally, the findings supporting the use of ipilimumab/nivolumab would be buttressed by external duplication in a second prospective clinical trial. This is especially important given the limited data on the efficacy of other agents in this setting. Another corroborative study could potentially establish ipilimumab/nivolumab as a new standard of care for post-PD-1/PD-L1 refractory MCC, but only for those patients likely to benefit from such treatment. However, in the absence of such data, while it appears reasonable to use ipilimumab/nivolumab for patients progressing on front-line anti-PD-1 therapy, the existing evidence is not yet substantial enough to formally designate this combination as the standard of care—a term that carries significant implications for both clinical practice and future research. Given that the majority of patients will not benefit from second-line ipilimumab/nivolumab therapy, there is an urgent need to develop new therapeutic strategies for these individuals.
In summary, effective options for second-line therapy in MCC remain limited, and there is a pressing need for innovative treatments to assist patients who do not respond to single-agent anti-PD-1 therapy. This underscores the ongoing necessity for clinical trials to explore and validate additional therapeutic strategies, aiming to enhance outcomes for this challenging patient population. The development of such strategies is vital not only to improve survival but also to offer quality of life benefits to those living with advanced MCC.
Bibliography
Appendix
Citation
@article{miller2024,
author = {Miller, David M.},
publisher = {Society of Cutaneous Oncology},
title = {Should {Ipilimumab} {Be} the {New} “{Standard}” for
{Refractory} {MCC?}},
journal = {Journal of Cutaneous Oncology},
volume = {2},
number = {1},
date = {2024-05-11},
url = {https://journalofcutaneousoncology.io/editorials/vol_2_issue_1/ipilimumab_for_pd1_refractory_mcc/},
doi = {10.59449/joco.2024.05.11},
issn = {2837-1933},
langid = {en}
}