Imaging Surveillance for Stage III Melanoma: Should We Be Stalkers or Casual Observers?
Featured Article
The Impact of Surveillance Imaging Frequency on the Detection of Distant Disease for Patients with Resected Stage III Melanoma. Dieng, Mbathio, Sarah J. Lord, Robin M. Turner, Omgo E. Nieweg, Alexander M. Menzies, Robyn P. M. Saw, Andrew J. Einstein, et al. 2022. Annals of Surgical Oncology 29 (5): 2871–81. https://doi.org/10.1245/s10434-021-11231-3.1
Introduction
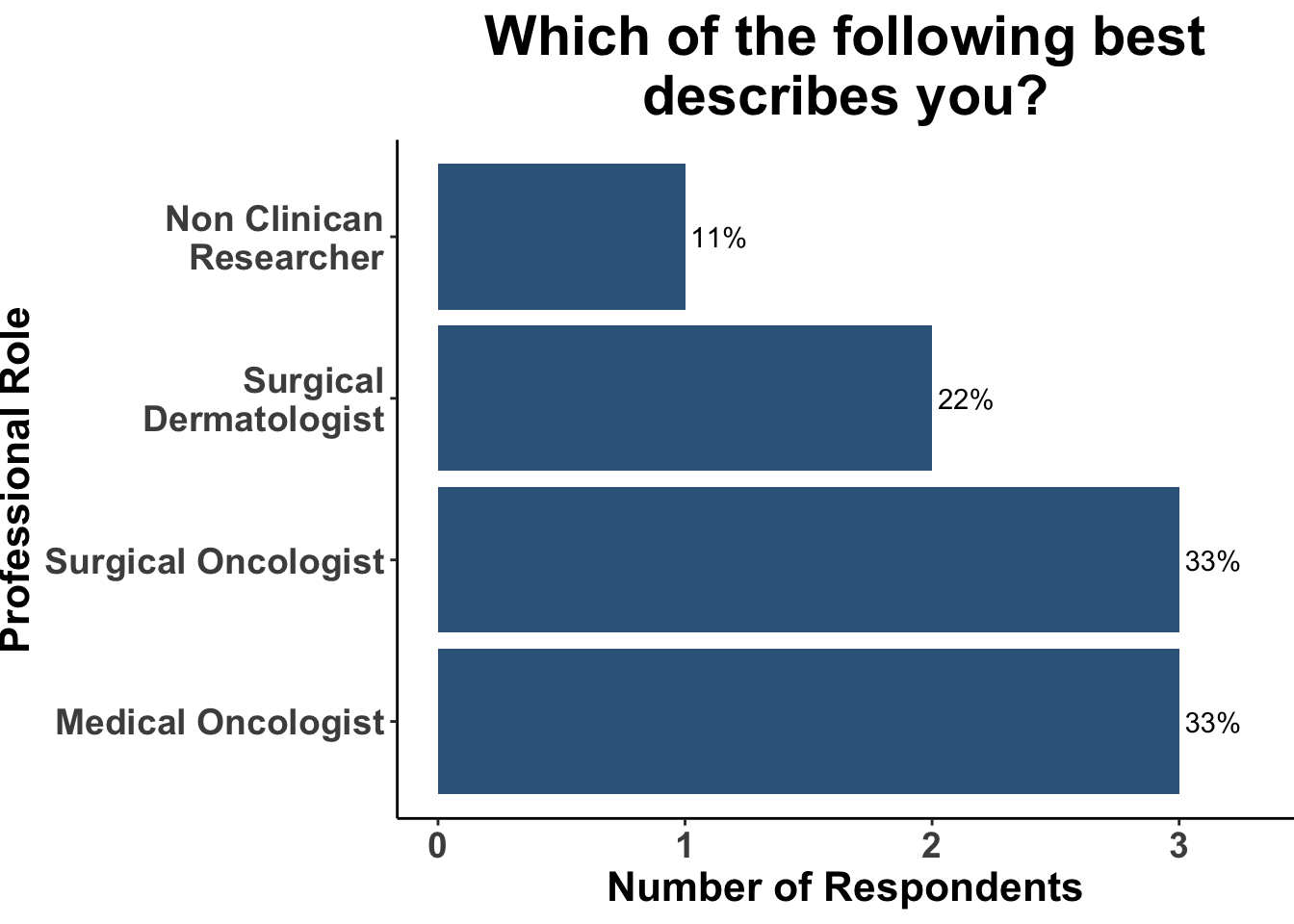
On July 10th, 2023 the multi-institutional Society of Cutaneous Oncology (SoCO) Journal Club (JC) reviewed the results of a recently published longitudinal cohort study that investigated the impact of frequency of surveillance imaging in patients with stage III melanoma.1 Journal club participants included clinicians and investigators from Massachusetts General Hospital, Massachusetts Eye and Ear, Brigham and Women’s Hospital, the National Institutes of Health, the Dana-Farber Cancer Institute, and the University of Sydney (Figure 1). This Perspectives on the Science article reflects the views of the authors after the Journal Club. Please note that it does not represent the views of any other members of SoCO or affiliated institutions.
Background
Imaging is a long-standing key component of surveillance in nearly all solid tumor malignancies. Very few cancers have serology markers that can better detect a recurrent tumor. While biomarker research and liquid biopsy development continue to progress, imaging is currently the standard and common practice for most cancers including melanoma for the near future. Despite imaging recommendations from National Comprehensive Cancer Network (NCCN) guidelines (Table 1), society guidelines, and institutional protocols there are no randomized trials to best define the optimal timing for surveillance imaging in melanoma. Therefore, observational studies must be relied upon to provide a reasonable amount of evidence to guide clinical practice. While there may be debate about the best imaging for surveillance, i.e. PET-CT vs. CT vs. MRI vs. US (ultrasound), the focus for this manuscript is on timing. This is an important question to consider as surveillance strategies can have significant impacts. Identification of recurrent tumor hopefully allows for treatment and cure, and therefore this is the key driver for surveillance. Early identification may also offer opportunities to intervene and limit symptoms related to tumor progression as well as provide valuable information for patients and families as they make important life decisions. However, surveillance imaging has many other obvious and more subtle potential impacts such as patient anxiety, medical visit fatigue, limits on imaging resources, cost to healthcare systems and limiting provider availability to name a few. Therefore, this is an important question for clinicians, patients, and health care systems.
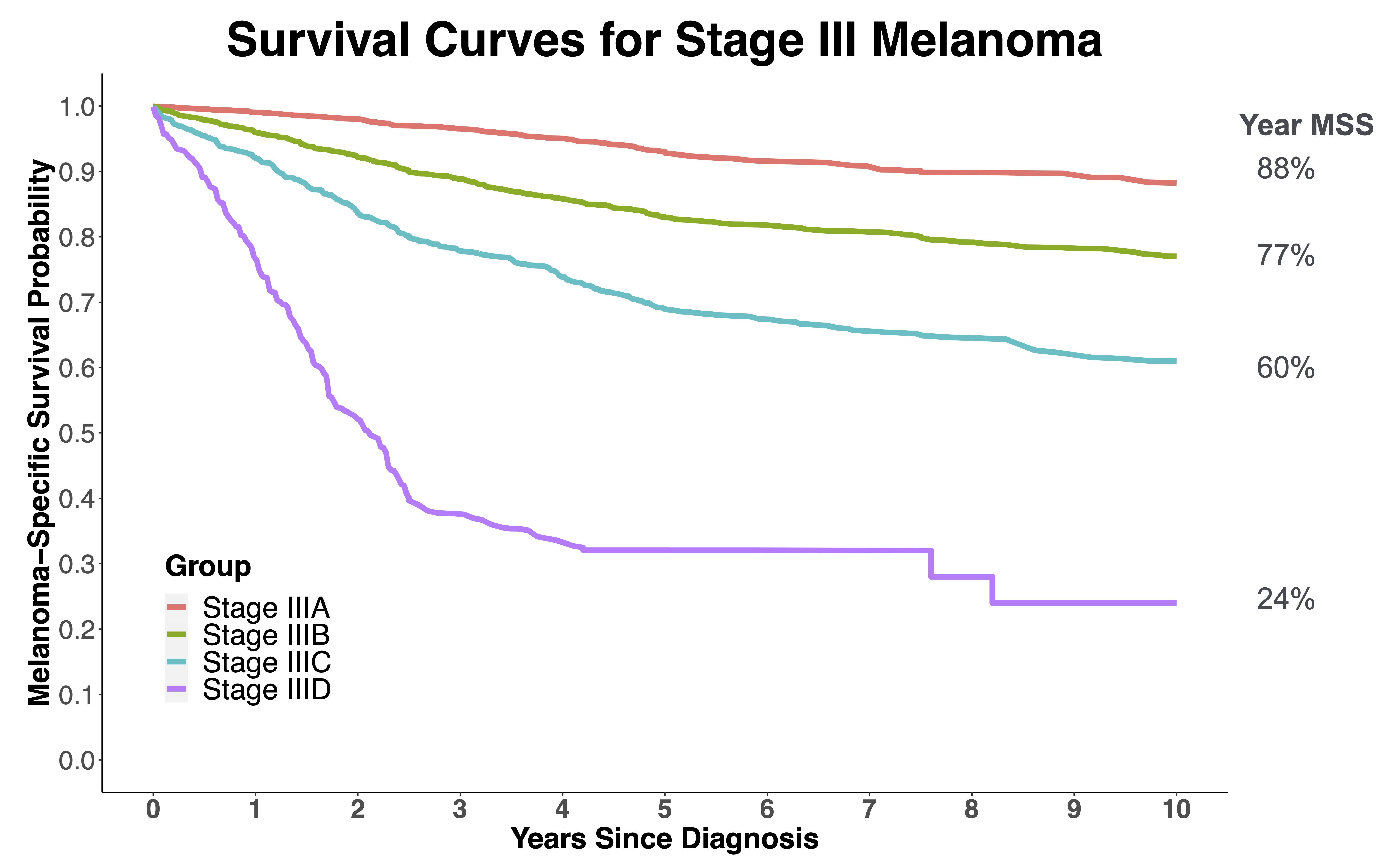
When developing surveillance strategies, it is also important to identify patients that are at highest risk for developing a recurrence and in particular recurrence that cannot be detected by patients or clinician physical exam. These patients are the most likely to benefit from intense surveillance. For melanoma, these recurrences are typically small lymph node recurrences or visceral metastases that would only cause symptoms at an advanced size / tumor burden. Stage III melanoma patients fit this profile well. While there is a significant difference in survival for stage IIIA vs. IIID (Figure 2),3 this is a good starting point for an observational study such as this.
However, when considering melanoma specifically, attention should also be given to stage IIC patients that have an equal or higher risk of distant metastasis than stage IIIa. It is also important to acknowledge that by defining who is at high risk, it also defines who is low risk and is very unlikely as a group to benefit from intense surveillance. Lastly, we should consider the term “high risk”. High risk of curable recurrence? Death? What risk level is considered high? This is a philosophical question for the medical community and general public, and it is currently not well defined. It may in fact vary from disease to disease depending on the lethality and morbidity of a recurrence or the treatments available should a recurrence be detected. As we contemplate surveillance imaging strategies these aspects are important to bear in mind.
There are a couple of additional key aspects to melanoma management that are important background for this paper. Systemic treatment for melanoma has rapidly evolved and improved over the past decade.4–8 The data in this study spans the decade prior to immune checkpoint inhibitor and BRAF targeted therapy regulatory approvals as well as the key period of clinical trials and first few years of rapid adoption after regulatory approvals. These developments are incredibly exciting and meaningful for patients but makes interpretation of data regarding natural history of the disease much more difficult. The last several years have also seen an important increase in the recognition of the significance of high risk and advanced non-melanoma skin cancers (NMSC). Since so much attention and resources are directed to melanoma, it is common for surgical and non-surgical melanoma treatments to be applied to NMSC. Therefore, it is quite likely approaches to surveillance such as those discussed in this manuscript could be applied to those cancers as well. It is critical that these diseases be recognized for their individual natural histories and that imaging surveillance strategies for melanoma with a high risk of distant visceral metastasis may not be applicable to advanced cutaneous squamous cell carcinoma with a high risk of local and regional metastasis and lower risk for distant metastasis.
Study Design
In the current study by Dieng et al.1 , the investigators performed a retrospective cohort study using the Melanoma Institute of Australia database and clinical trials files. This is one of the largest prospectively collected datasets and is well curated and managed. The inclusion group was comprised of stage IIIA-D patients who underwent complete surgical resection of their regionally metastatic disease. All patients included had baseline imaging prior to surgery and surveillance was determined by clinician preference or clinical trial protocol if enrolled. From this dataset three groups were created based on the imaging frequency: every 3- to 4- months, every 6 months, and every 12 months. The primary objective was to examine these three groups with the intent to define the proportion of patients with diagnosed distant recurrence as a result of surveillance imaging, distant disease-free survival (DDFS), melanoma-specific survival (MSS), post distant recurrence MSS (dMSS), and overall survival (OS). Kaplan-Meier was used to describe survival outcomes at 2- and 5- survival rates. Log-rank test was used to assess survival differences. Subgroup analyses were run to assess differences in survival based on imaging frequency.
Main Findings
The study enrolled 473 patients that met the inclusion criteria between 2000 and 2017. CT was the dominant form of imaging (85%). 30% of patients were in the 3- to 4- month group, 10% in the 6- month group and 60% in the 12- month group. Reflective of clinician’s concern for stage IIIC and IIID patients, these patients made up 66% of the 3- to 4- month group 62% of the 6-month group and 41% of the 12- month group. Development of distant metastasis in this cohort was similar to reported experiences based on stage of disease (Fig 1 in manuscript1). Interestingly there was an equal number of distant metastasis identified on imaging (40%) as identified on physical exam (43%). The remaining were identified by other imaging modalities not part of the surveillance plan (Table 2 in manuscript1).
The analysis revealed that the MSS was actually shortest in the 3- to 4- month interval follow up group. Importantly, the median post dMSS for the entire group was 3.6 years. The stage IIIC group had a significantly shorter median post dMSS at 11.6 months for the 3- to 4- month follow up group and 16 months in the 12- month follow up group. For those with stage IIIB disease the post dMSS was the same in both the 3- to 4- month and 12 month follow up groups.
Discussion
At the heart of surveillance imaging and at the core of this manuscript is the question of whether identifying a metastasis earlier with a more frequent imaging schedule would improve survival once that metastasis is detected. Not surprisingly, in the high-risk stage IIIC group metastasis were found earlier when using 3- to 4- month interval. However, this did not lead to a survival advantage. This is perhaps the key take home from the presented data. There appears to be equipoise between shorter and longer interval imaging approaches relative to dMSS. However, this data set does not tease out some of the potential other important impacts that we mentioned in the introduction. For example, did more frequent imaging lead to more or less anxiety for patients? What was the total cost to the healthcare system between these groups? Were oncology teams better able to focus on patient visits and patient needs with less frequent imaging? It was also striking to see the frequency in which distant metastasis were identified on physical exam, reinforcing the importance of in person visits and not simply relying on imaging.
Before dismissing the potential impact on improved survival with shorter interval surveillance imaging approaches, it is critical to remember the context of the time frame of this study. The end of this study time frame captures the earliest moments of the introduction of immune checkpoint inhibitors and BRAF targeted therapy. These are now widely available and routinely used. One could hypothesize that with effective systemic treatments now available earlier identification of metastasis would lead to improved survival. However, this question is more complex because it is unknown whether or not these systemic treatments are more effective at a lower volume of disease, i.e. disease detected at an earlier time point. It may be that these systemic treatments are equally effective regardless of stage disease is identified. This will be a very difficult question to answer but could have an important impact on how surveillance imaging is approached in the future.
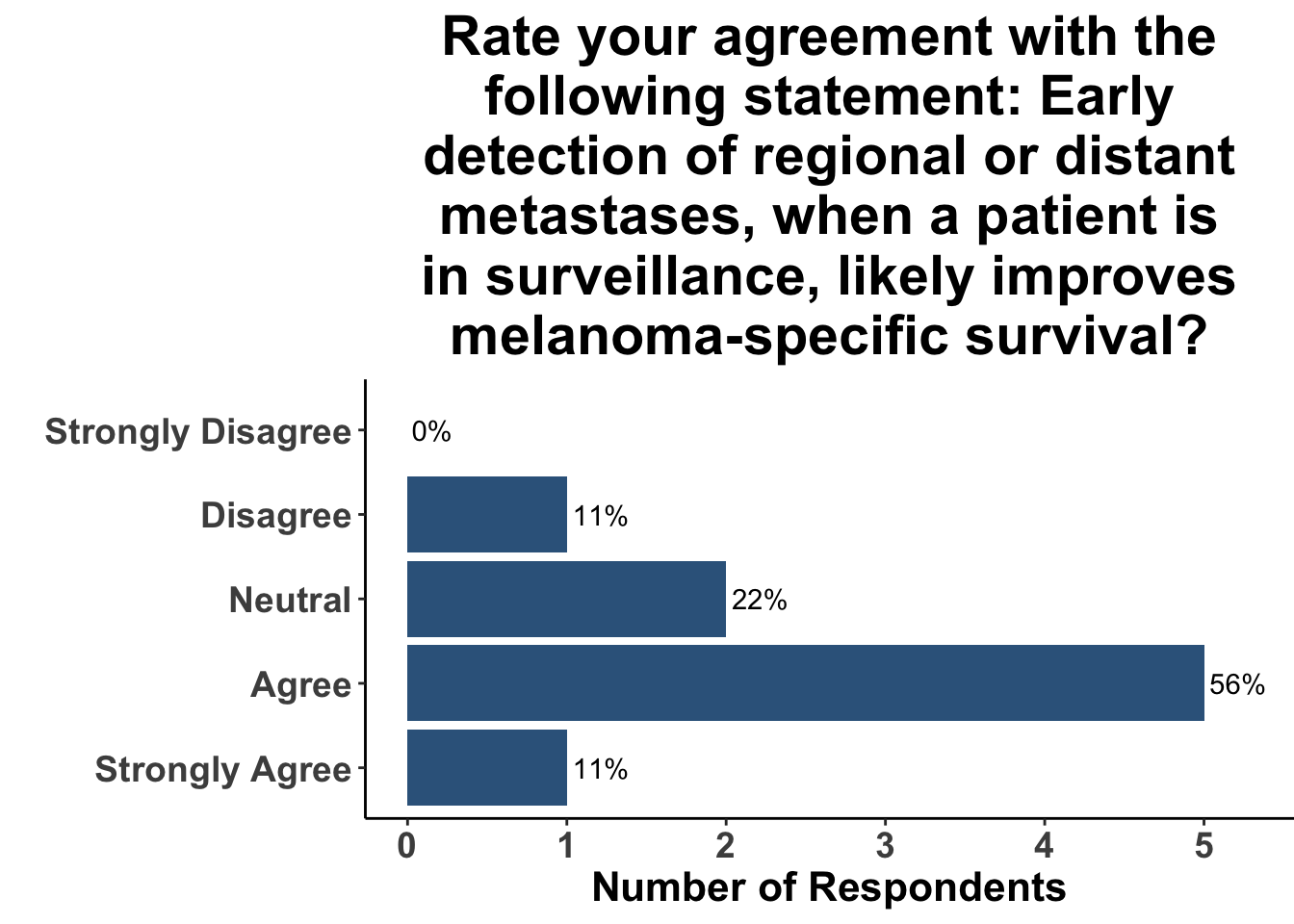
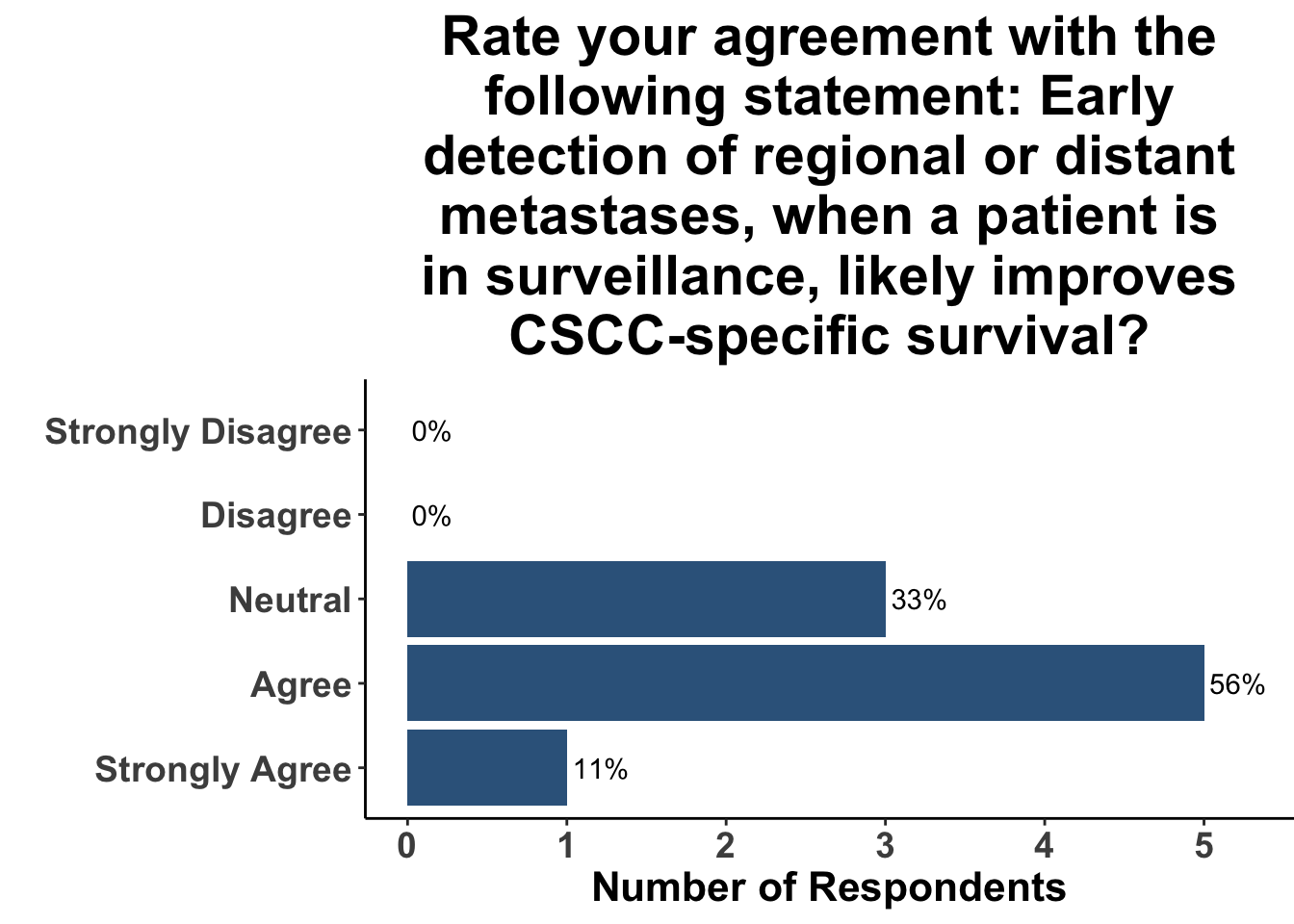
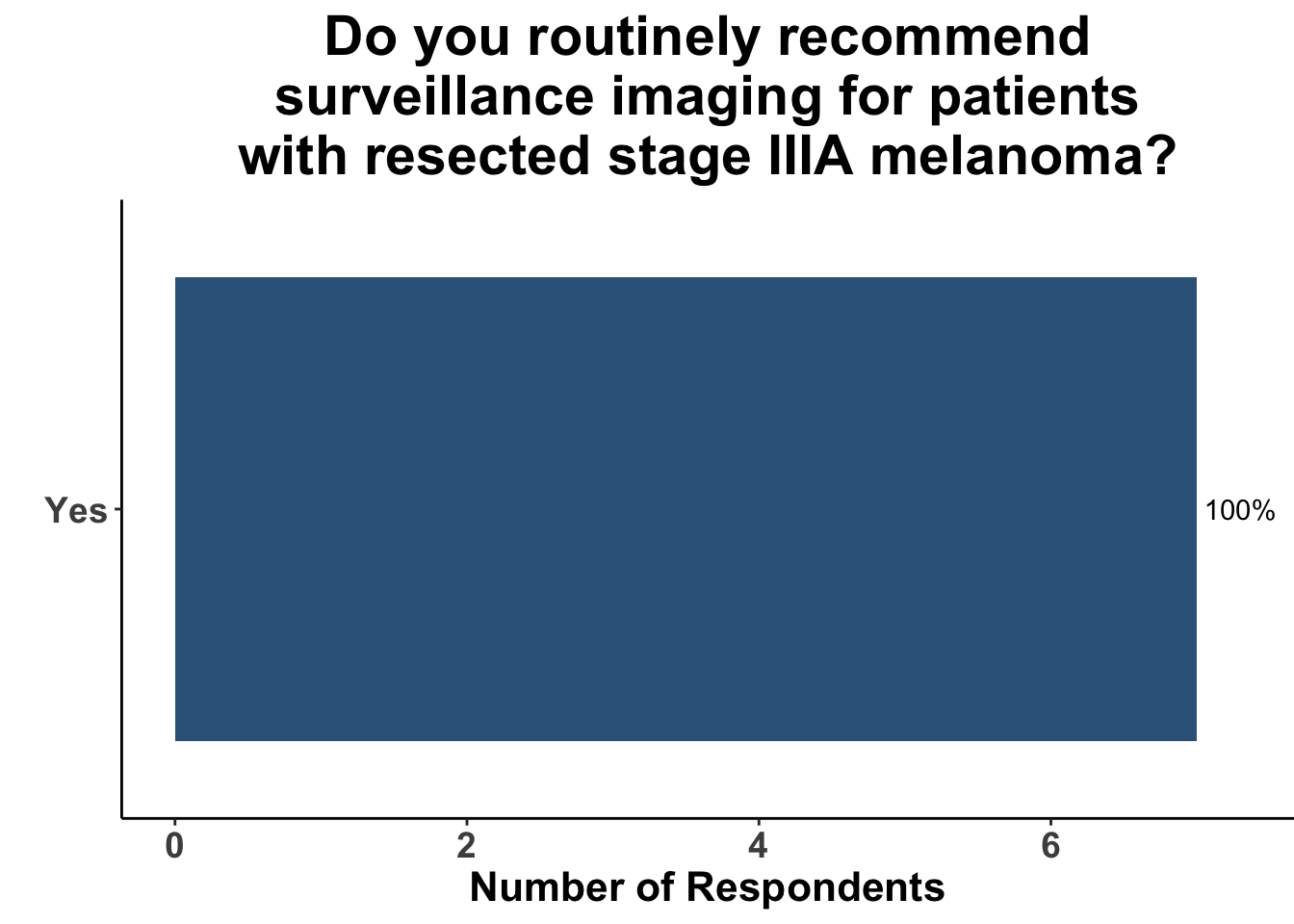
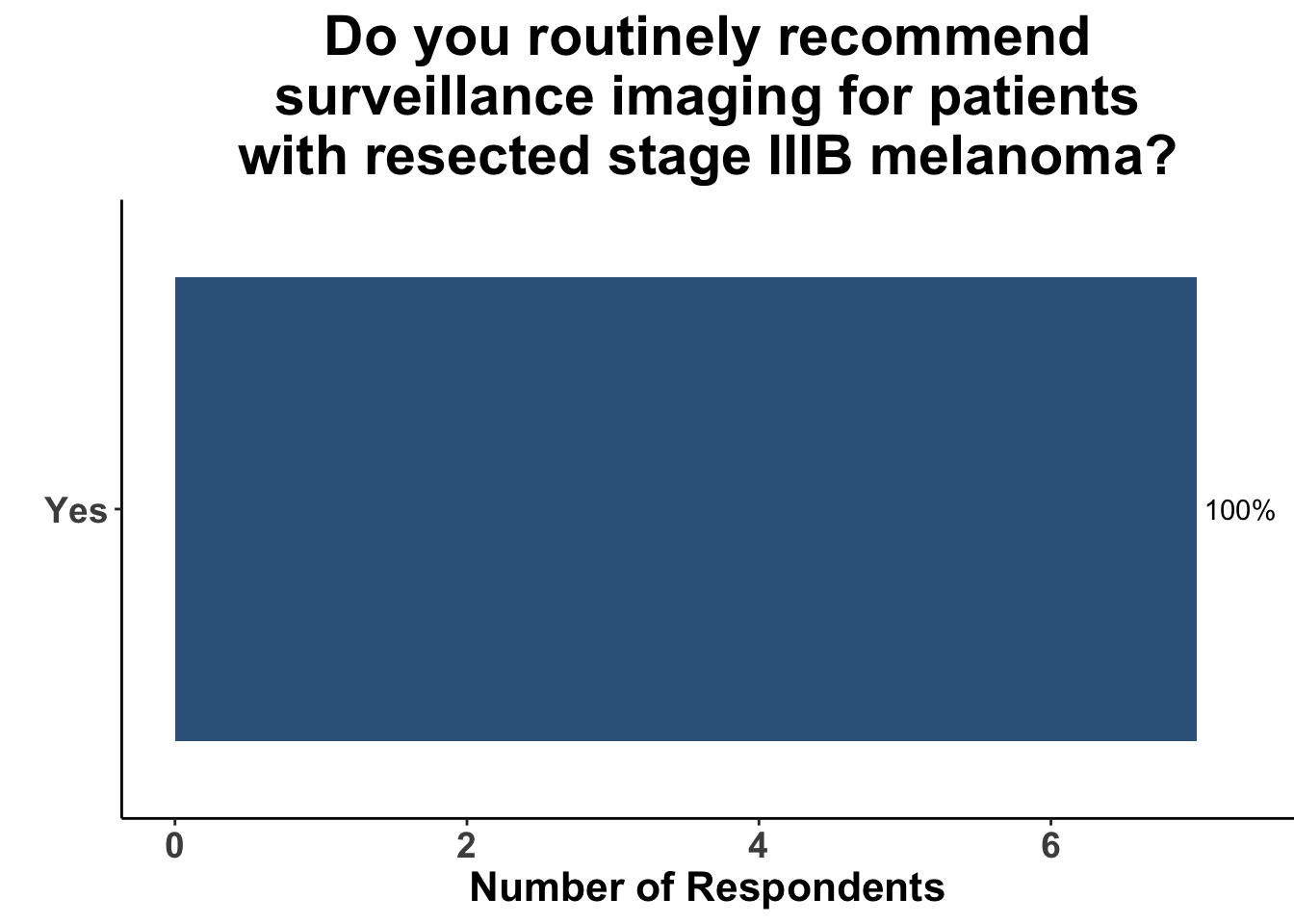
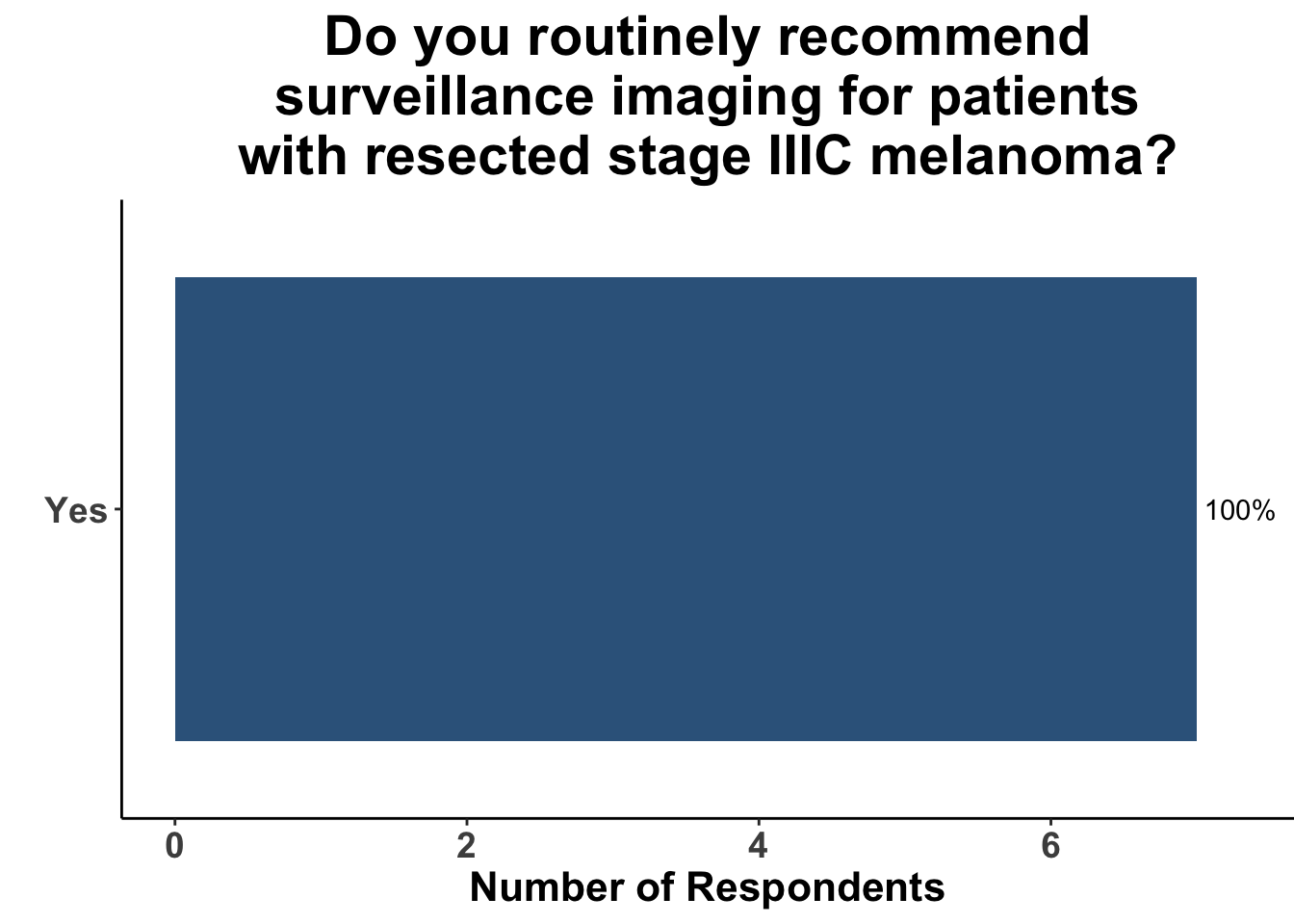
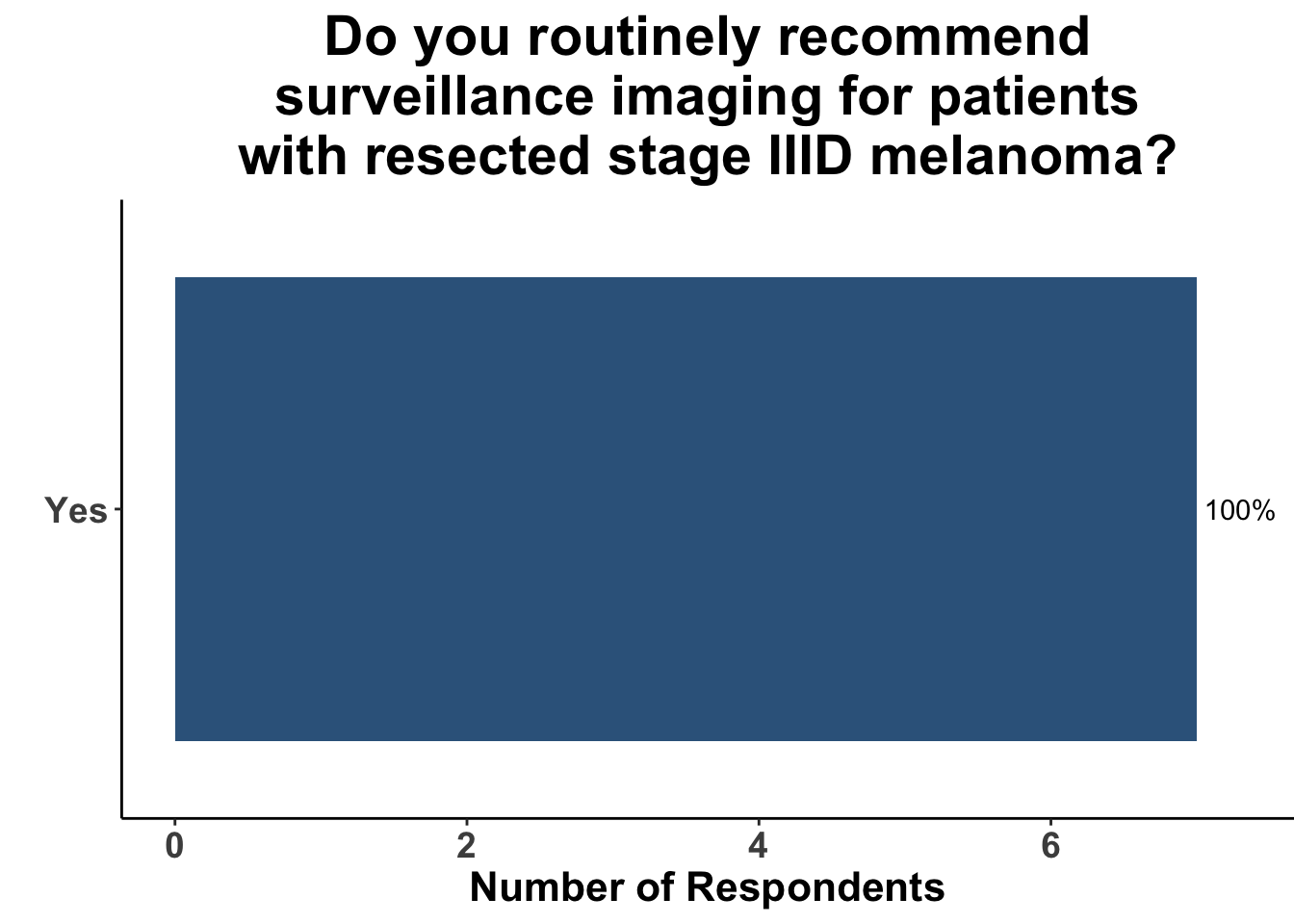
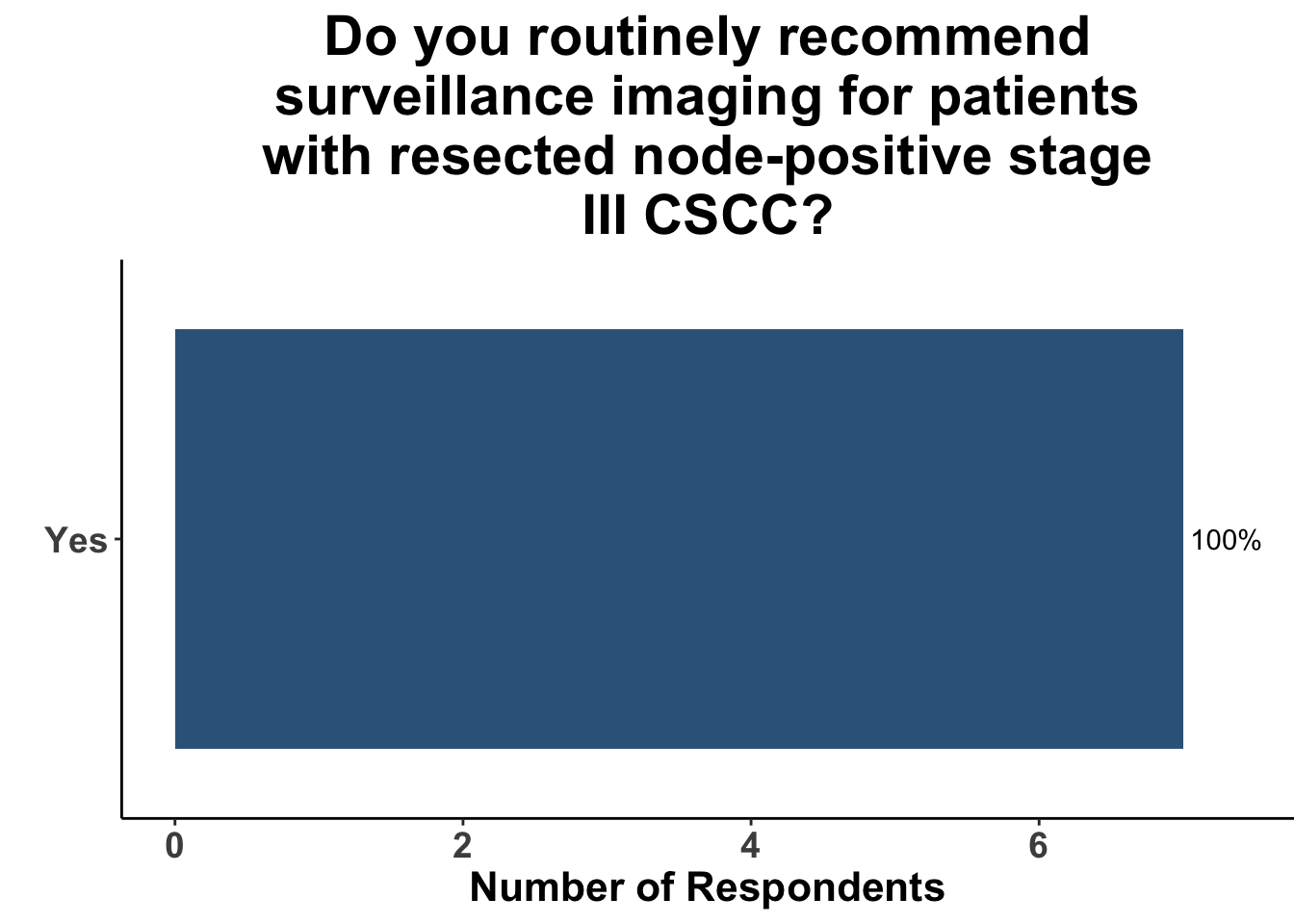
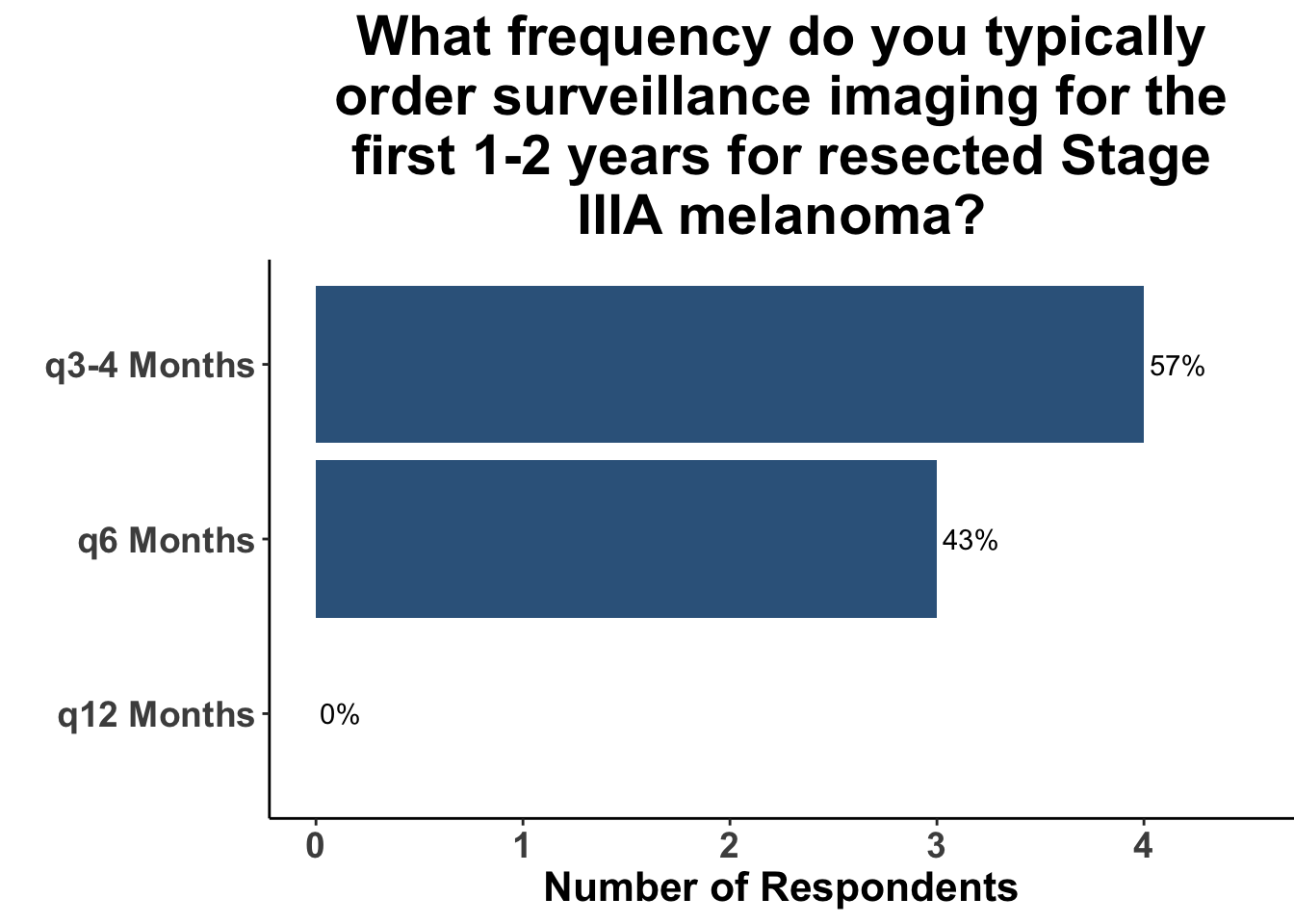
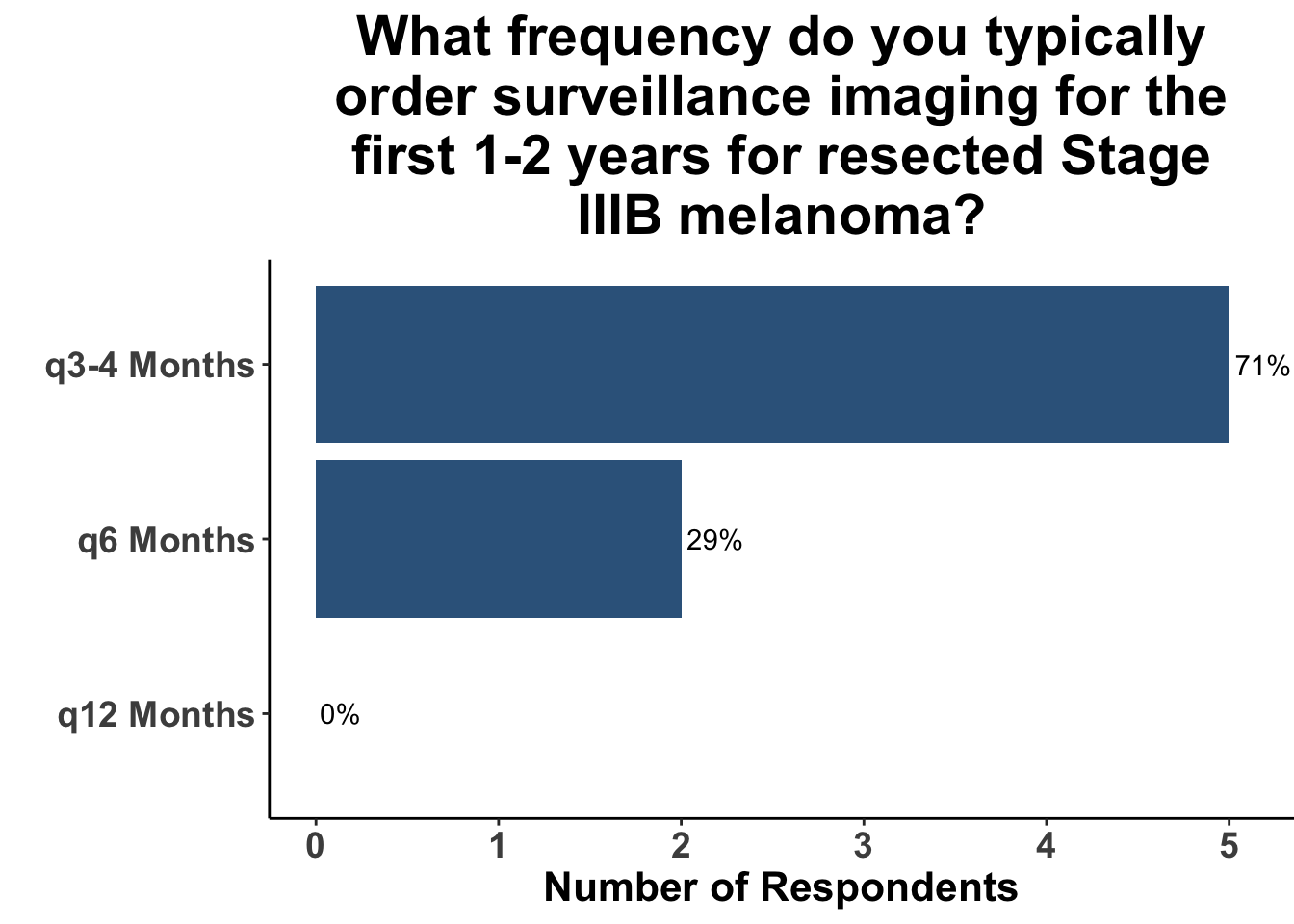
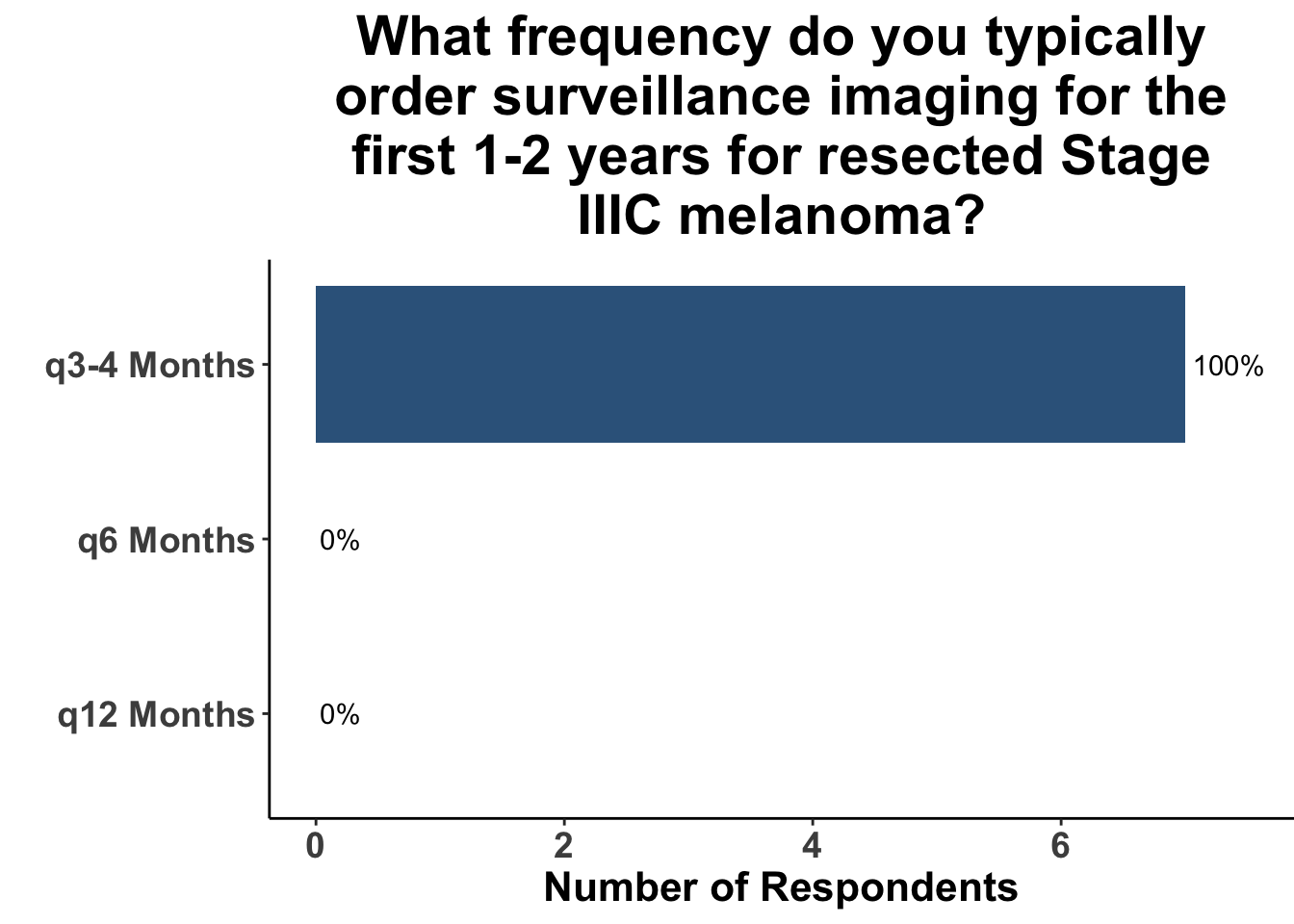
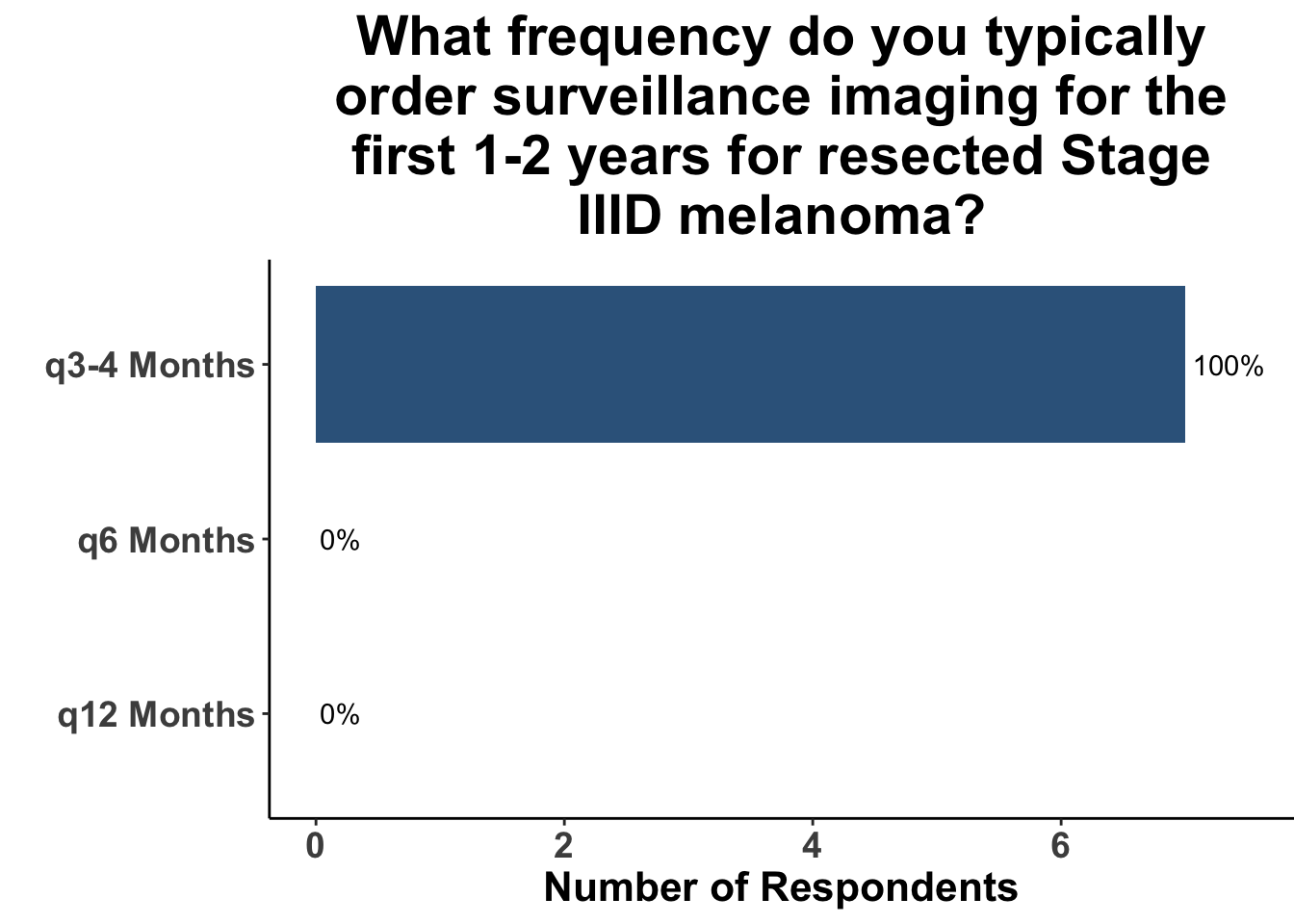
Given some of the limitations of this paper we sought to provide some real-world context by polling our July 10th SoCO journal club attendees about their experiences with imaging for both melanoma and NMSC. In line with the philosophy mentioned above, 67% of participants either agreed or strongly agreed that early identification of distant metastasis would improve MSS (Figure 3) and identification of regional and distant metastasis would improve cSCC disease-specific survival (DSS) (Figure 4). This is reflected in the group’s real-world experience that everyone recommends routine surveillance imaging for stage III A-D melanoma patients (Figure 5, Panels A-D). Similarly for patients with node positive stage III cutaneous squamous cell carcinoma (CSCC) all clinician respondents recommended routine surveillance imaging (Figure 6).
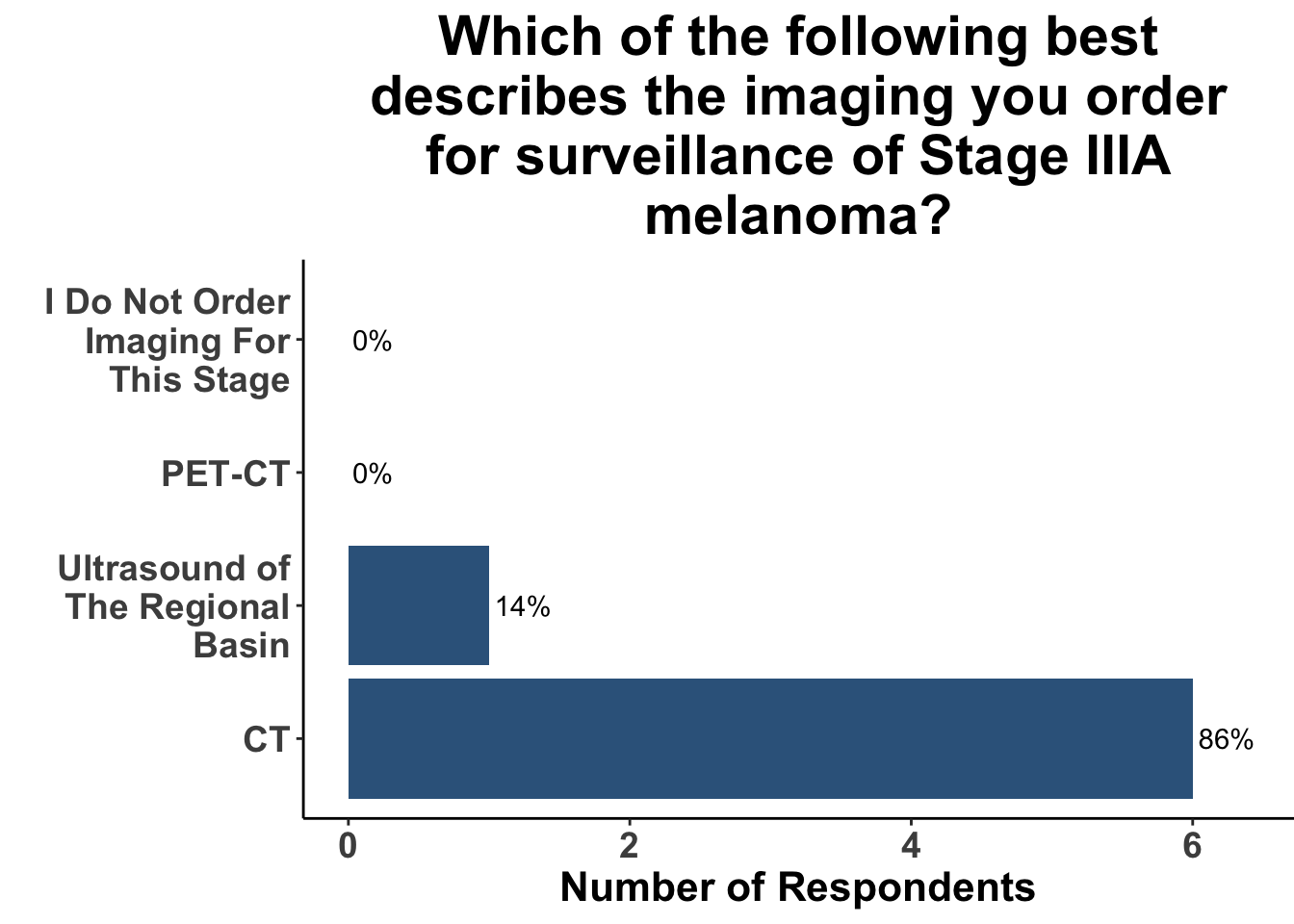
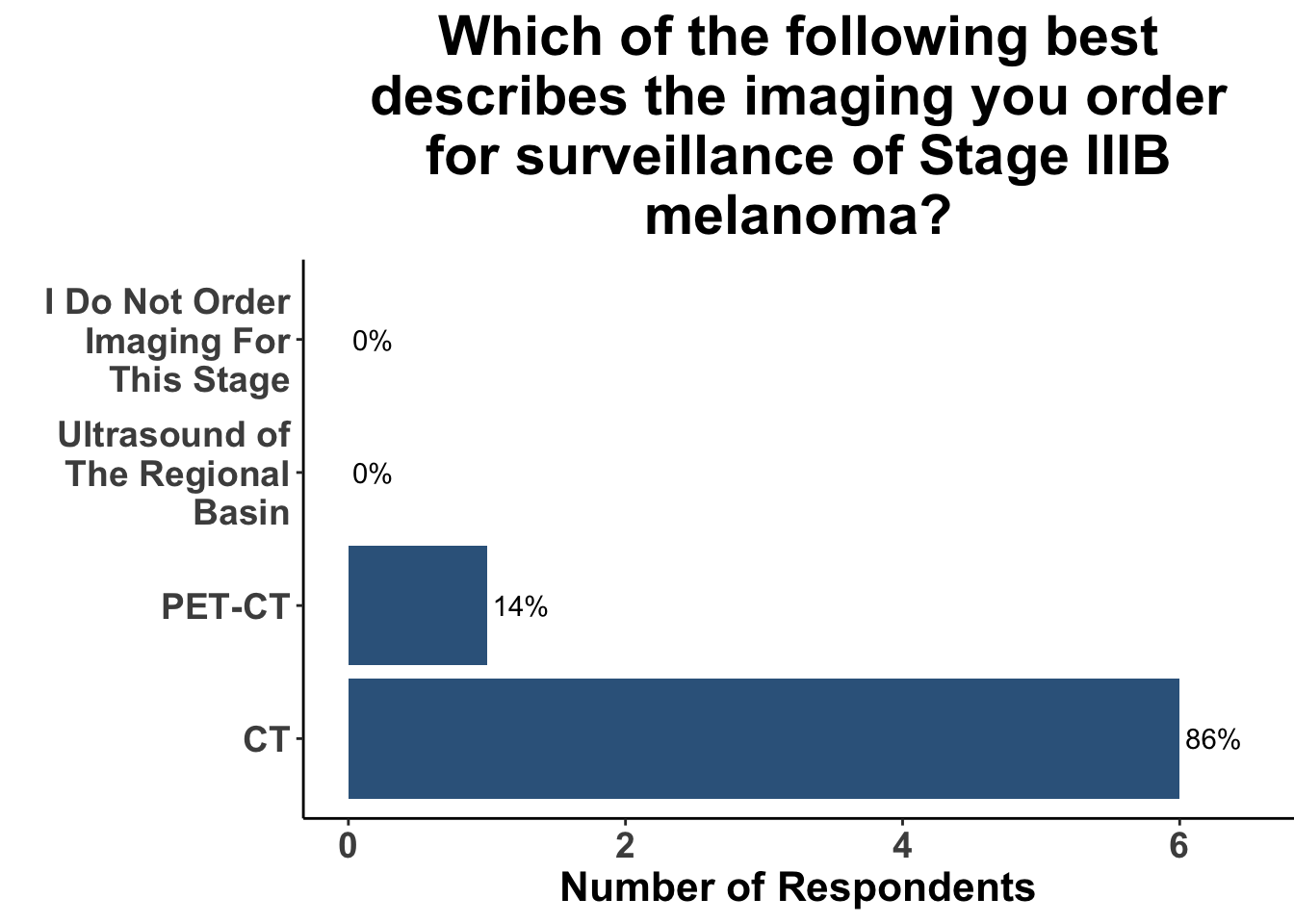
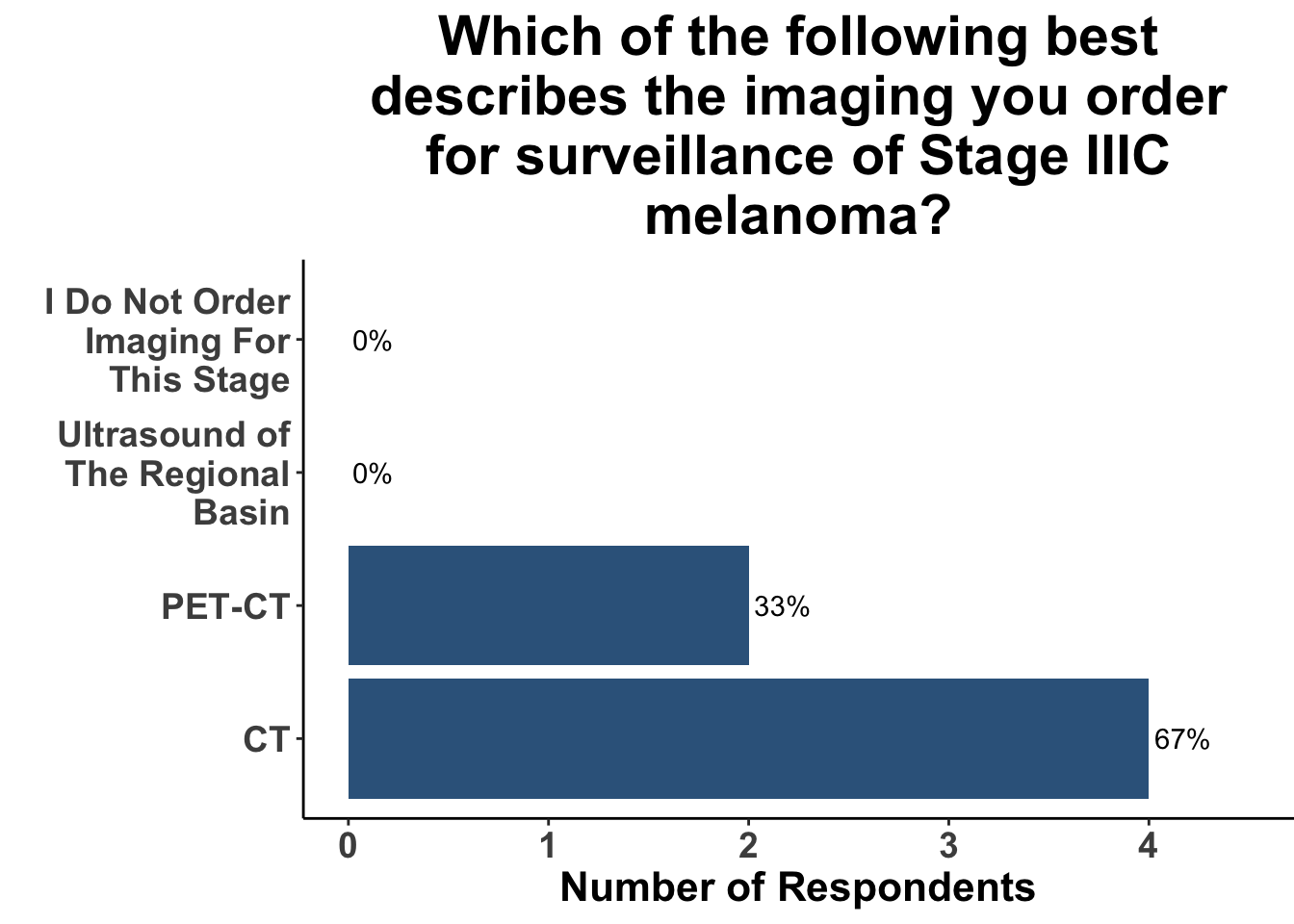
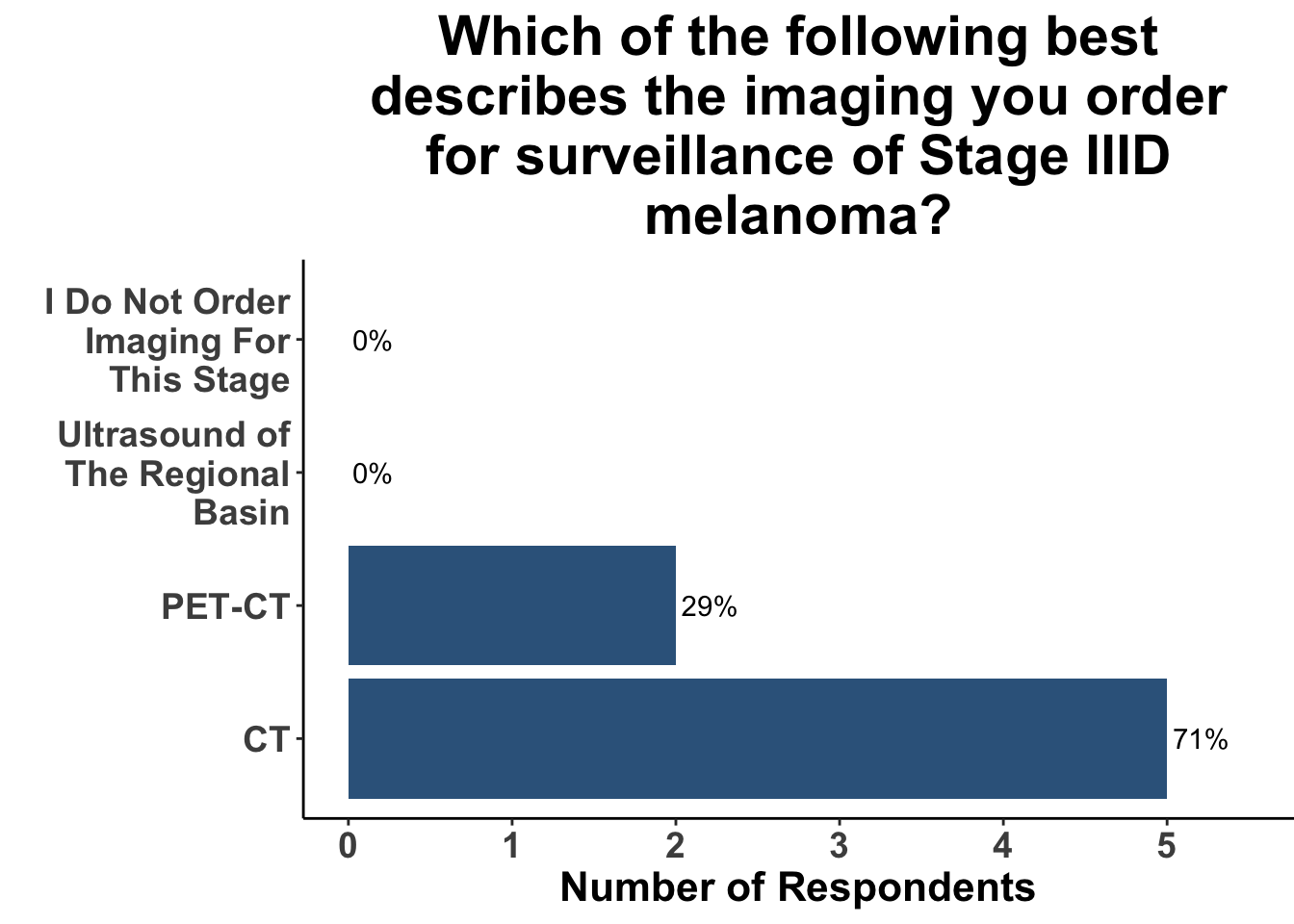
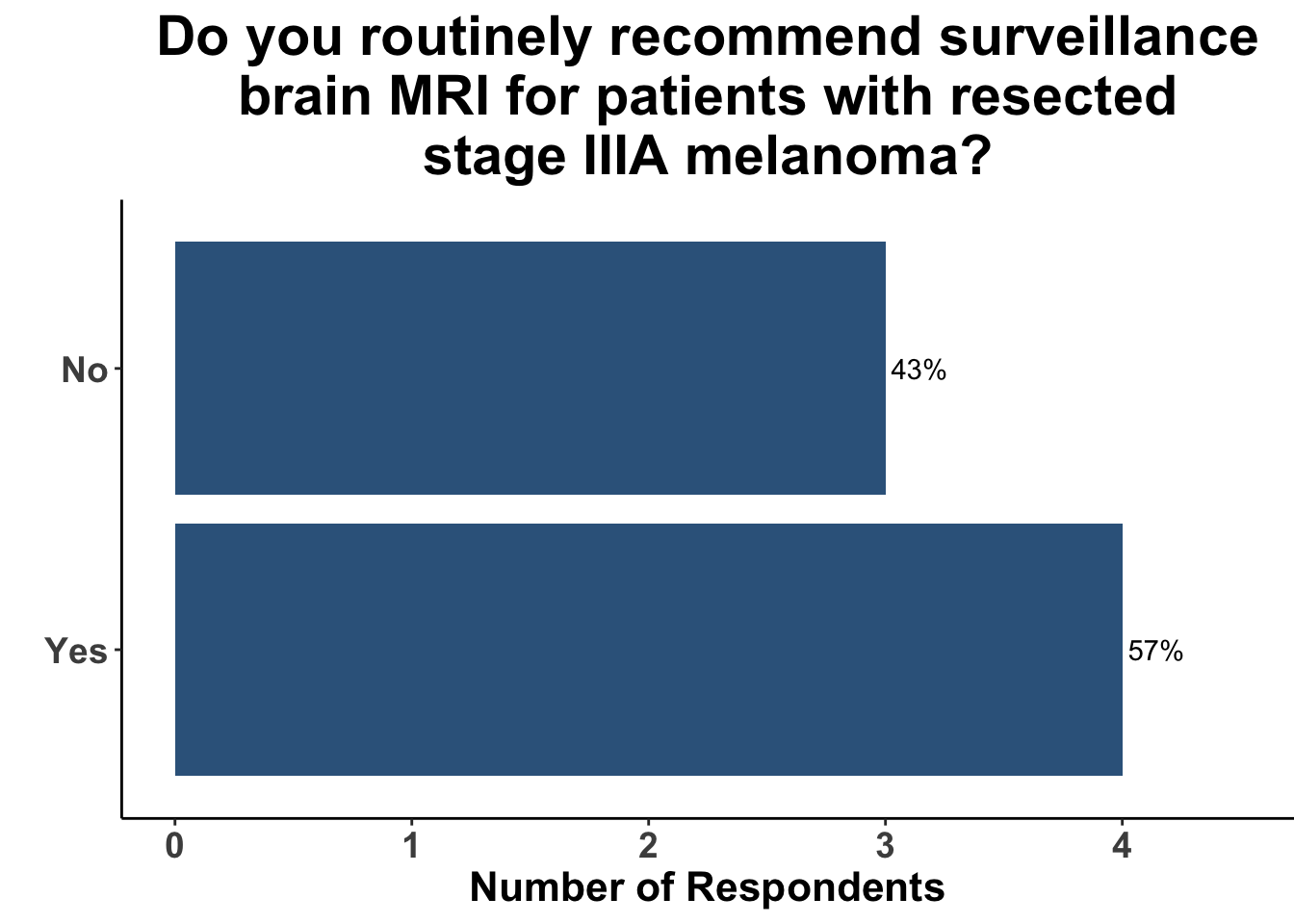
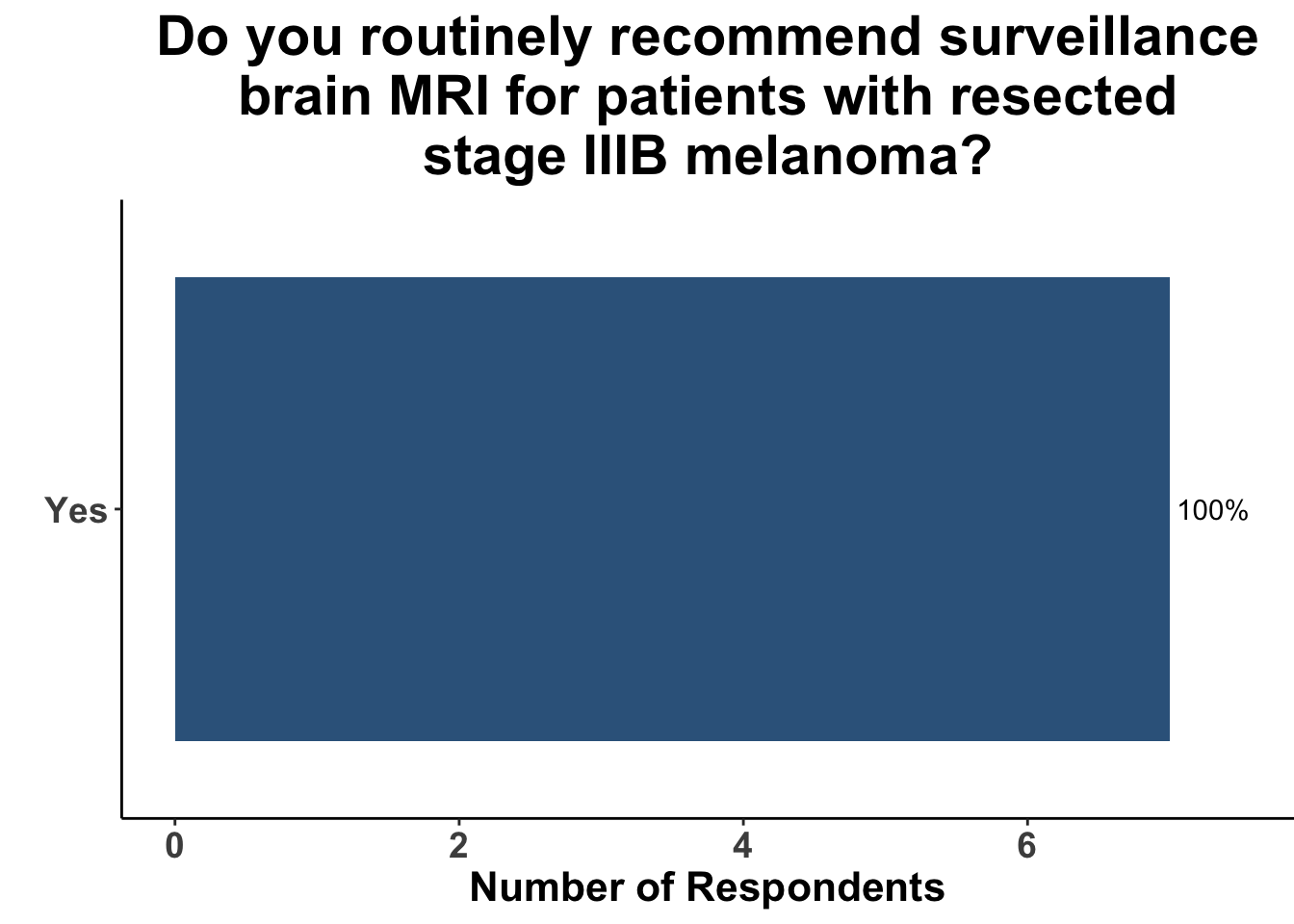
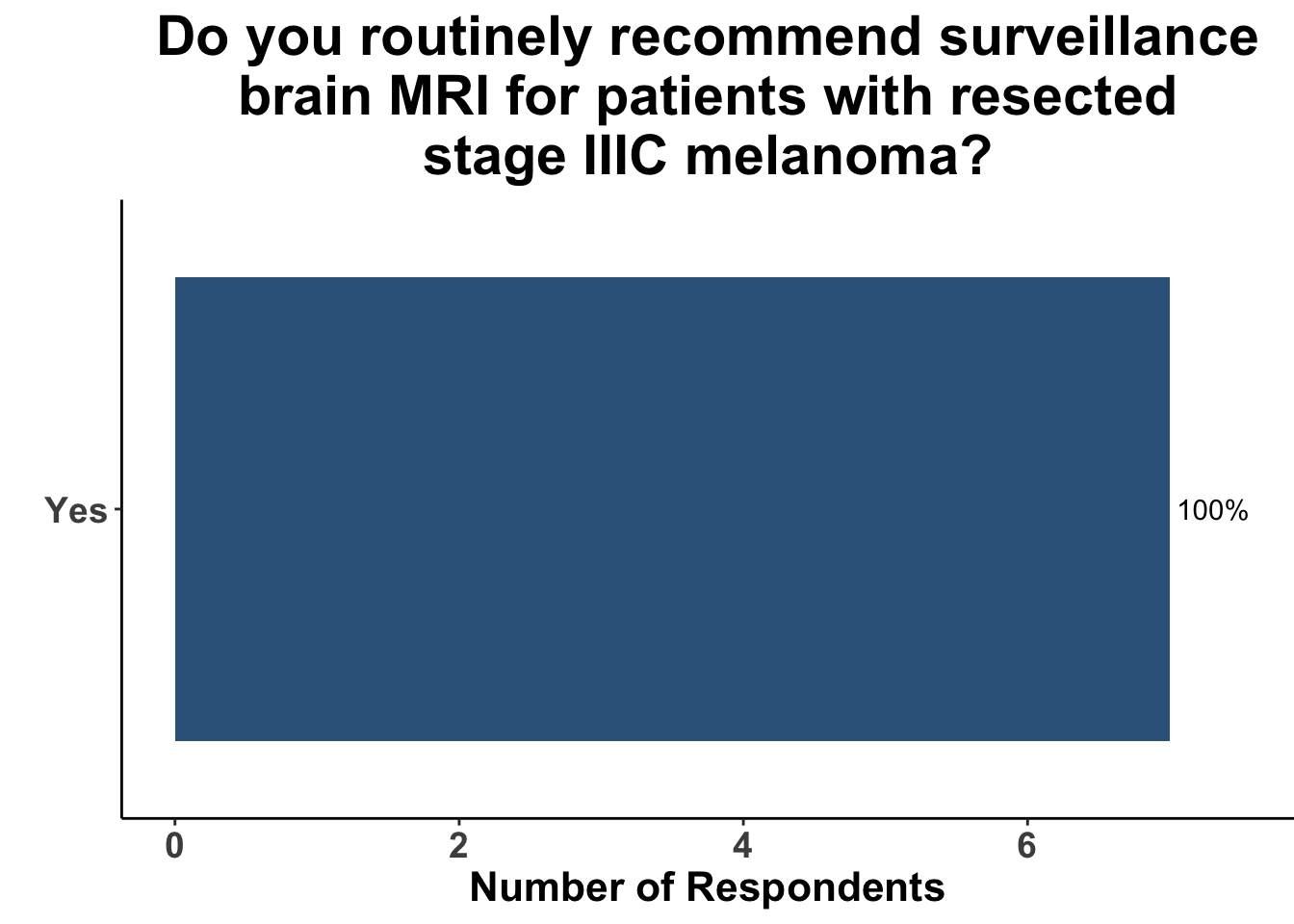
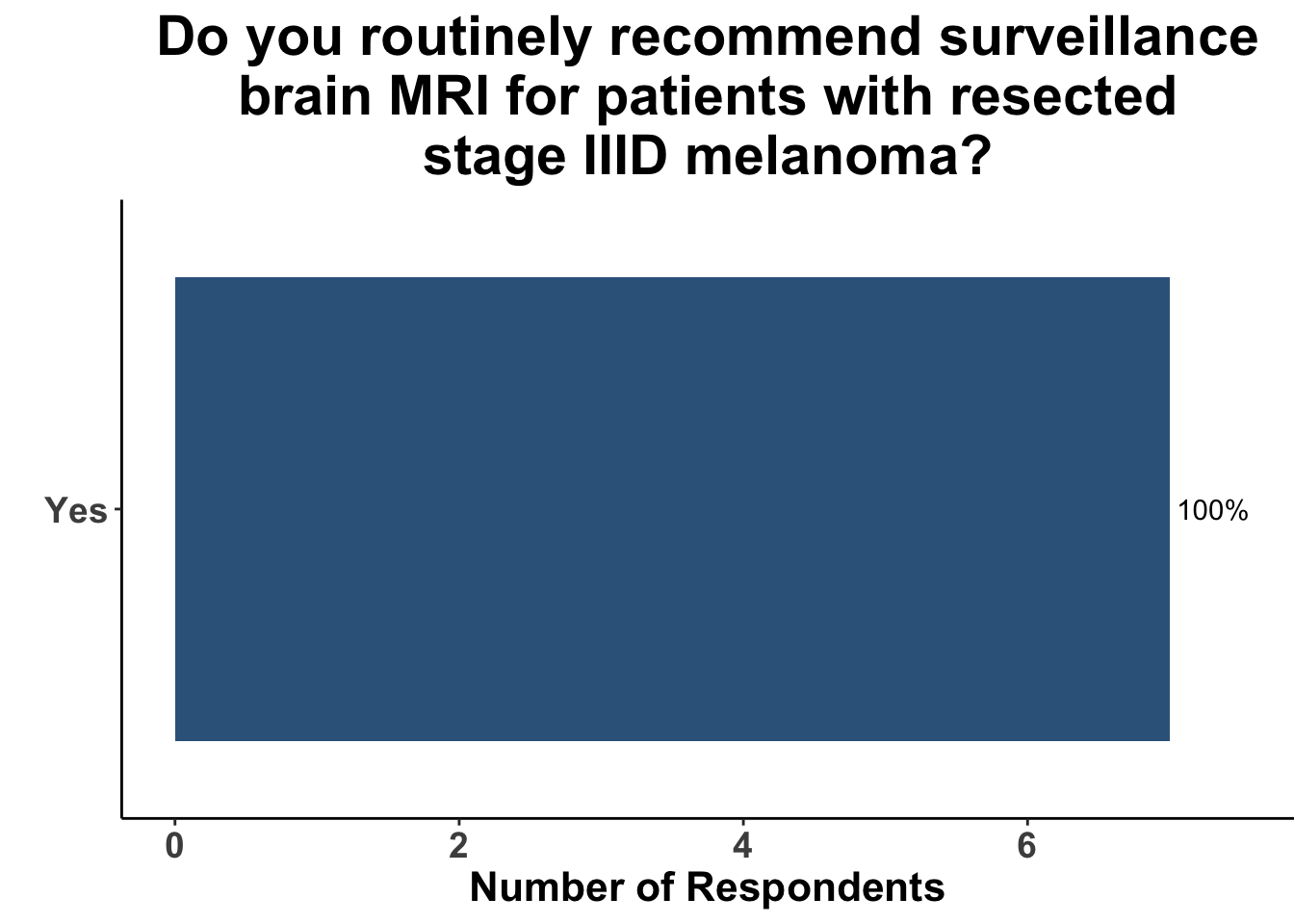
This study included PET-CT as well as diagnostic cross-sectional CT. Imaging modality was determined based on physician preference as well as clinical trial protocols. Therefore, we asked our SoCO attendees what their real-world approach is to imaging in stage III melanoma patients. For each subgroup of stage III disease, CT imaging was preferred (Figure 7). However, there did appear to be a trend towards increasing use of PET-CT for stage IIIC and D patients. We also queried the group about their use of brain MRI given the potential for brain metastasis not captured on CT or PET-CT. All respondents signaled they obtain a brain MRI for stage III B-D patients as part of their routine surveillance (Figure 8).
In terms of timing, which was the key question in this manuscript, the group favored a 3- to 4- month imaging plan. This was particularly true for stage IIIC/D patients. All responding clinicians reported this frequency as their current imaging timing approach. The lower risk of distant metastasis in stage IIIA was reflected in participants response that one third commonly used a 6- month approach. 20% of respondents use a 6- month approach in stage IIIB. As a comparison, we also queried the common practice for node positive squamous cell carcinoma. These responses were much more heterogeneous with more providers leaning toward 3- to 4- month protocols, but an almost equal number utilizing a 6- month approach.
The attendee responses are quite revealing about the overall state of imaging surveillance for our advanced stage skin cancer patients. It is clear from the presented data that there is not an obvious improvement in survival with more frequent imaging with the caveat that current applications of systemic treatments may alter this outcome. Nonetheless, among this group of attendees it appears that for stage III melanoma most clinicians still prefer an every 3-4 months surveillance strategy. This approach may still be providing important non-survival benefits to patients but also poses additional challenges including cost and logistical burdens for the healthcare system and providers. These are important real-world factors that need to be considered as we look to find and improve data in the future that will better guide skin cancer oncology teams.
Conclusion
Surveillance imaging is important in the management of advanced skin cancer patients. This paper provides an important data point suggesting that imaging on a more frequent basis does not improve survival outcomes for stage III melanoma patients. However, this type of observational cohort needs to be replicated in a period that includes the regular use and availability of effective systemic treatments. It is clear from the participants at the SoCO journal club that more frequent imaging continues to be the preferred approach. Future investigations into this issue need to look at additional factors beyond the typical survival metrics used in most studies.
Materials and Methods
This Perspectives on the Science piece was published using Quarto®. The survey was conducted using REDCap®.9 The figures depicting the survey data were created using R (version 4.0.0) and the tidyverse suite of packages,10 including ggplot2.11 The image on the “Perspectives on the Science” page was created by the authors (DMM) using the rosemary package.12
Bibliography
Appendix
Citation
@article{emerick2024,
author = {Emerick, Kevin S and Miller, David M.},
publisher = {Society of Cutaneous Oncology},
title = {Imaging {Surveillance} for {Stage} {III} {Melanoma:} {Should}
{We} {Be} {Stalkers} or {Casual} {Observers?}},
journal = {Journal of Cutaneous Oncology},
volume = {2},
number = {1},
date = {2024-02-14},
url = {https://journalofcutaneousoncology.io/perspectives/vol_2_issue_1/imaging_surveillance_for_stage_iii_melanoma/},
doi = {10.59449/joco.2024.02.14},
issn = {2837-1933},
langid = {en}
}