Adjuvant anti-PD-1 for Merkel Cell Carcinoma: Ready for the Clinic?
PlumX
Altmetric
Featured Article
Becker, J. C. et al. Adjuvant immunotherapy with nivolumab versus observation in completely resected Merkel cell carcinoma (ADMEC-O): disease-free survival results from a randomised, open-label, phase 2 trial. The Lancet (2023) doi:10.1016/s0140-6736(23)00769-9.
Introduction
On August 4th, 2023 the multi-institutional Society of Cutaneous Oncology (SoCO) Journal Club (JC) reviewed the results of the recently published ADMEC-O trial.1 Participants included clinicians and investigators from Massachusetts General Hospital, Massachusetts Eye and Ear, Brigham and Women’s Hospital, the National Institutes of Health, the Dana-Farber Cancer Institute, and the University of Sydney (Figure 1). This Perspectives on the Science article reflects the views of the authors after the Journal Club. Please note that it does not represent the views of any other members of SoCO or affiliated institutions.
Background for the Study
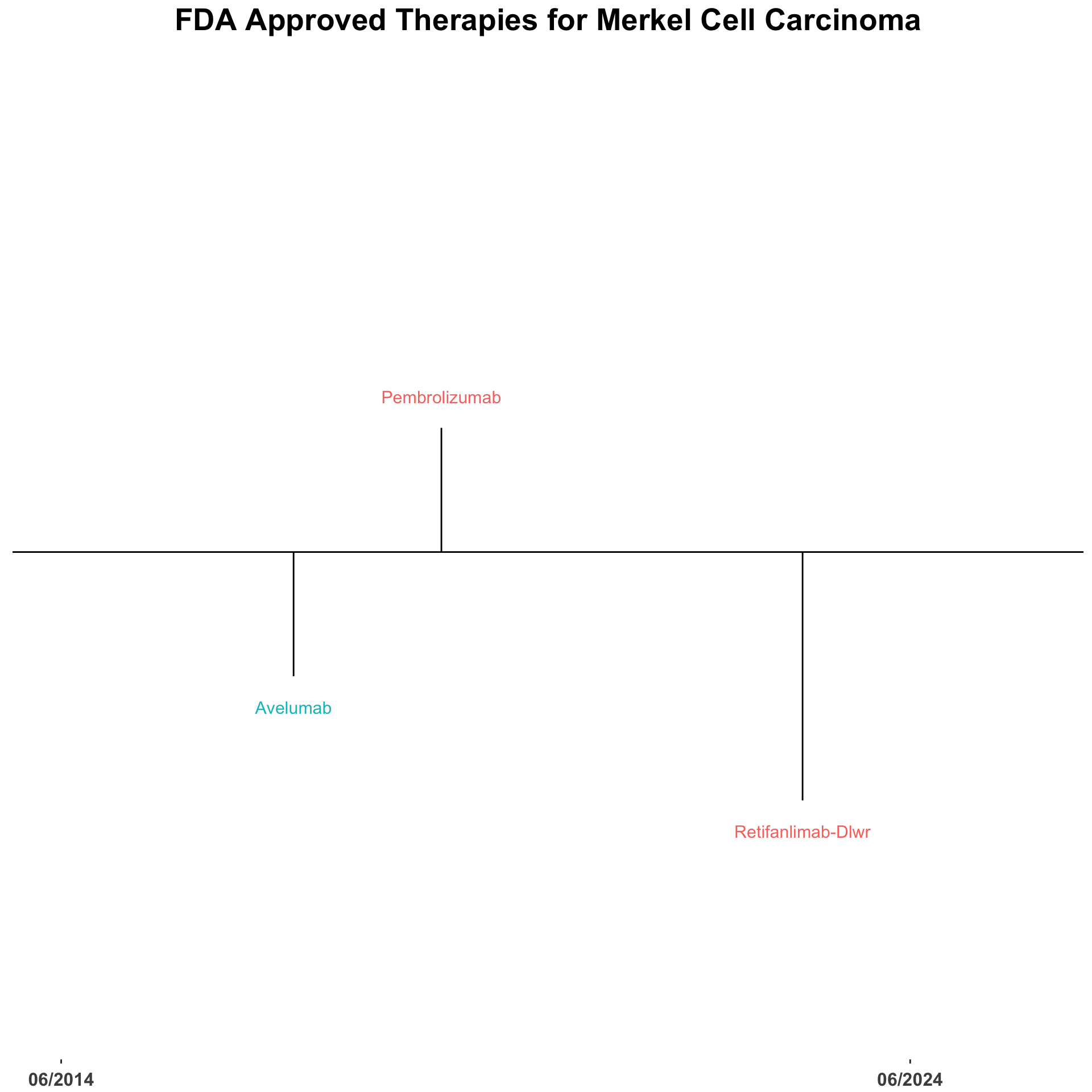
The treatment landscape for advanced Merkel cell carcinoma (MCC) has changed dramatically over the last decade. The utilization of immune checkpoint inhibitors (ICI) for patients with locally advanced or metastatic disease has improved the clinical outcomes for many patients.2–4 There are currently three FDA-approved anti-PD-1/PDL-1 monoclonal antibodies approved for the use in the advanced setting (Figure 2).5 In addition, data from several trials have demonstrated activity of monotherapy nivolumab and combination nivolumab with ipilimumab comparable to the FDA-approved agents (Table 1).6–15
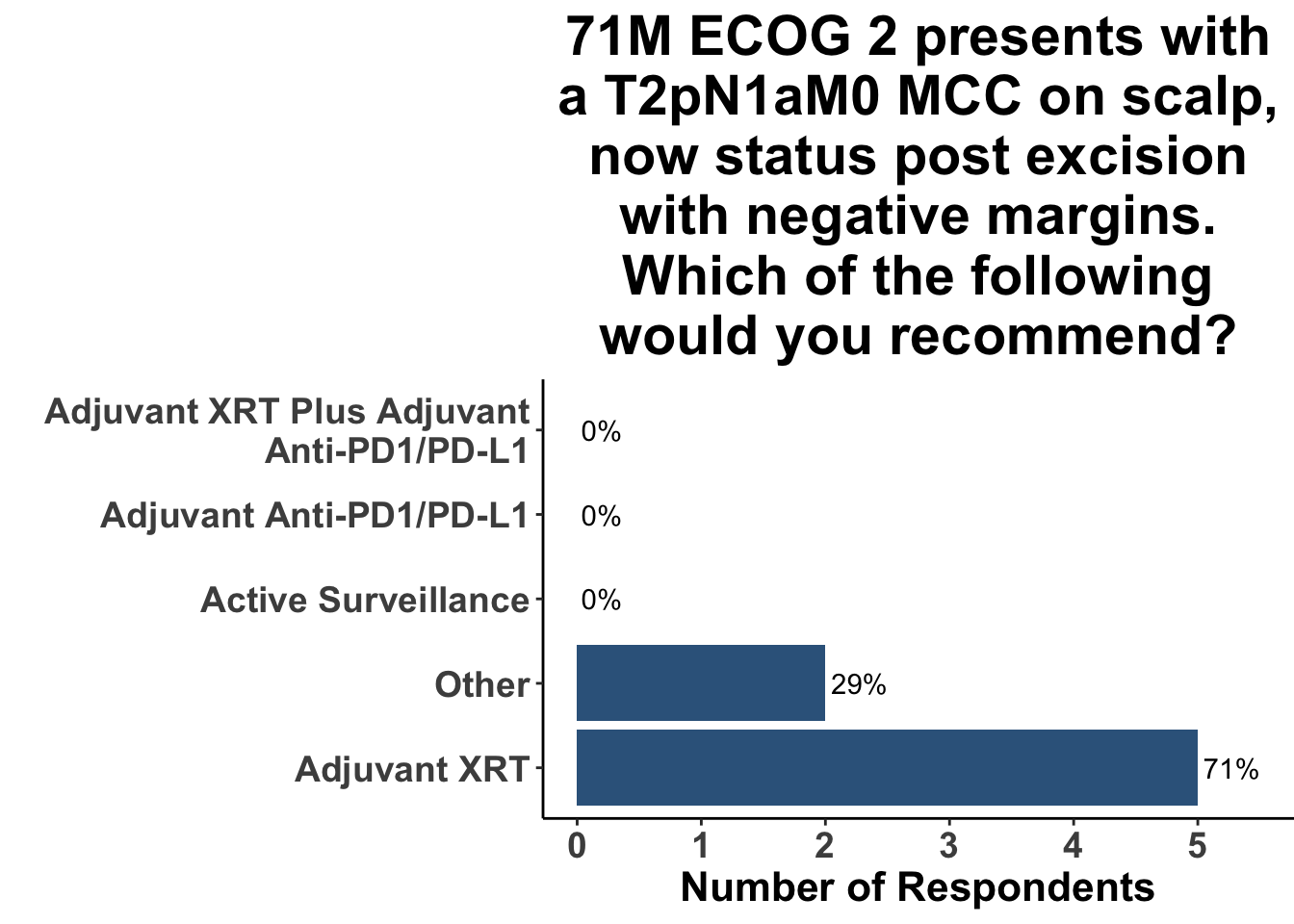
In contrast, the treatment landscape for resectable disease has evolved less substantially. Surgical excision followed by post-operative radiotherapy is commonly used for the management of skin-only and microscopic-nodal disease. As an example, 71% of the clinical participants at the August 4th SoCo Journal Club responded that they would recommend post-operative radiation therapy for a patient with Stage II MCC (Figure 3). Given the benefit of immunotherapy in advanced MCC, along with the success of ICI in the high-risk resectable (HRR) setting in melanoma,16–20 numerous clinic trials have been developed for fully-resected MCC (NCT02196961, NCT03712605, NCT04291885, NCT03271372).
One of the challenges in designing prospective clinical trials in the post-operative setting, however, is the lack of prospectively-collected epidemiological data. In MCC, natural history data are derived from a combination of large administrative databases, such as the National Cancer Database (NCDB),21 as well as single-institution data sets.22,23 Information on critical endpoints, such as disease-free survival (DFS), are omitted from some of these sources. Outcomes data from the NCDB, which lacks information on DFS, was used to establish the current American Joint Committee on Cancer (AJCC) staging system for MCC21. In the 8th-edition AJCC, the expected 5-year overall survival (5yr-OS) for skin-only disease was 50.6%, with 5yr-OS rates of 45% for clinical stage I and 62.8% for pathological stage I (Table 2). More recently, however, published series from high-volume centers have provided data that demonstrate both better outcomes,22,23 as well as expanded data on outcomes such as DFS (Table 3).23
Given these limitations, many of the studies conducted over the last 10 years have relied on data either from the 8th-edition AJCC data set - or in the case of the ADMEC-O trial, even earlier retrospective studies - for their projections.
Study Design
In the current study by Becker et al.1, the investigators evaluated the association of post-operative nivolumab with MCC recurrence in a multi-center phase 2 trial in 20 academic centers in Germany and the Netherlands. Patients with completely resected disease of any stage were randomly assigned at a ratio of two-to-one to receive 480 mg of nivolumab every 4 weeks for 1 year or observation. Randomization was stratified by stage, age, and sex. Landmark DFS was the primary endpoint. Sample size calculations were based on an assumption that DFS at 1 year for patients randomized to the control group would be 62%. Therefore, to show an increase to 80% in the treatment group with a power of 80% at a significance level of 0.05, 159 patient would be needed. Given an assumed drop out rate of 10%, the investigators planned to enroll 177 patients for the study.
Study Results
The study enrolled 179 patients between October 1, 2014 and August 31, 2020. 118 patients were randomized into the nivolumab group and 61 into the observation group. For those patients randomized into the nivolumab group, 12-month and 24-month DFS rates were 85% and 84%, respectively. 12- and 24-month DFS rates for those randomized into the observation group were 77% and 73%, respectively. Overall survival data were not mature. Grade 3-4 adverse events were reported in 42% of patients who received at least one dose of nivolumab and 11% of patients in the observation group. The absolute risk reduction in 12-month DFS was 0.091 (95% CI –0.017 to 0.212) and 0.100 for 24-month DFS (95% CI –0.019 to 0.237). The relative risk reduction at 12 months was 0.388 (95% CI –0.184 to 0.659) and 0.384 (95% CI –0.183 to 0.649) at 24 months. The Cox proportional hazard ratio (HR) was 0.58 (0.30-1.12). The 12- and 24-month DFS for patients with stage 1-2 disease was 94.1% at both time points for those treated with nivolumab and 82.0% in the observation group. The 12- and 24-month DFS for patients with stage 3-4 disease was 81.2% and 79.8%, respectively, for those treated with nivolumab. Those managed with observation exhibited 12-month DFS rate of 74.0% and 24-months DFS rates of 67.3%.
Discussion
Although MCC treatment options have increased for patients with locally advanced and unresectable disease, options in the post-operative, fully resected setting remain limited. Consequently, this is an active area of investigation with several clinical trials currently underway evaluating the role of anti-PD1/PD-L1 monotherapy in patients with high-risk disease. The ADMEC-O trial is the first among these to report their results.
The investigators should be commended for their efforts; this is an important contribution to the field. The study is evaluating a high-valued clinical question. These interim data suggest that nivolumab connotes clinical benefit in decreasing the risk of recurrence for patients with fully-resected disease. Notwithstanding the lack of statistical persuasiveness, the findings are biologically plausible. Immune checkpoint inhibitors induce responses in over half of patients with advanced MCC. While extrapolating from other diseases is fraught with caveats, activity in the advanced setting has translated into decreases in recurrences when immunotherapy is given in the postoperative setting in melanoma. This confluence of factors enhances the confidence in the ADMEC-O’s findings. Additional data from the ADAM and STAMP trials will likely provide further clarity on the activity of ICI in adjuvant MCC.
Yet challenges persist. The major limitation of ADMEC-O is its small sample size, given the observed rate of recurrence. Consequently, the study was considered exploratory and the trial’s primary endpoint was described as descriptive; a formal hypothesis test was not performed. Therefore, a conclusive statistical inference cannot be drawn. Further complexity arises from nuances in study design, in particular, disparities in post-surgical interventions between the intervention and control groups. Specifically, patients in the observation group received post-operative radiation at a higher frequency compared to the intervention group (74% vs. 50%). In retrospective observational studies, adjuvant radiation has been associated with improved outcomes.24,25 Therefore this difference in post-surgical treatment confounds our ability to know the true effect of adjuvant nivolumab. While it is conceivable that higher rates of adjuvant radiotherapy in the observation group may have lead to lower recurrences, perhaps biasing the study towards the null hypothesis, this supposition should be weighed judiciously.
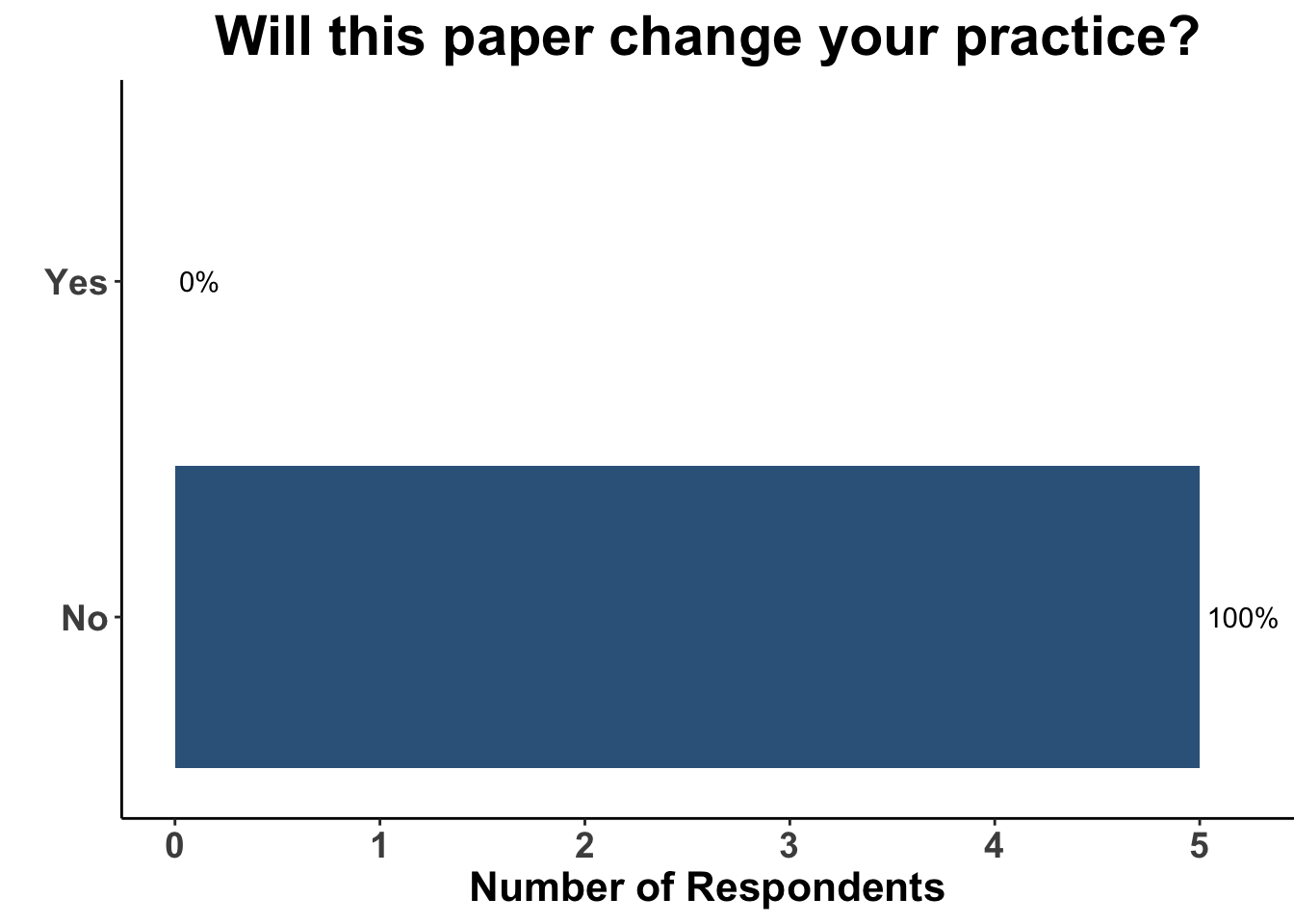
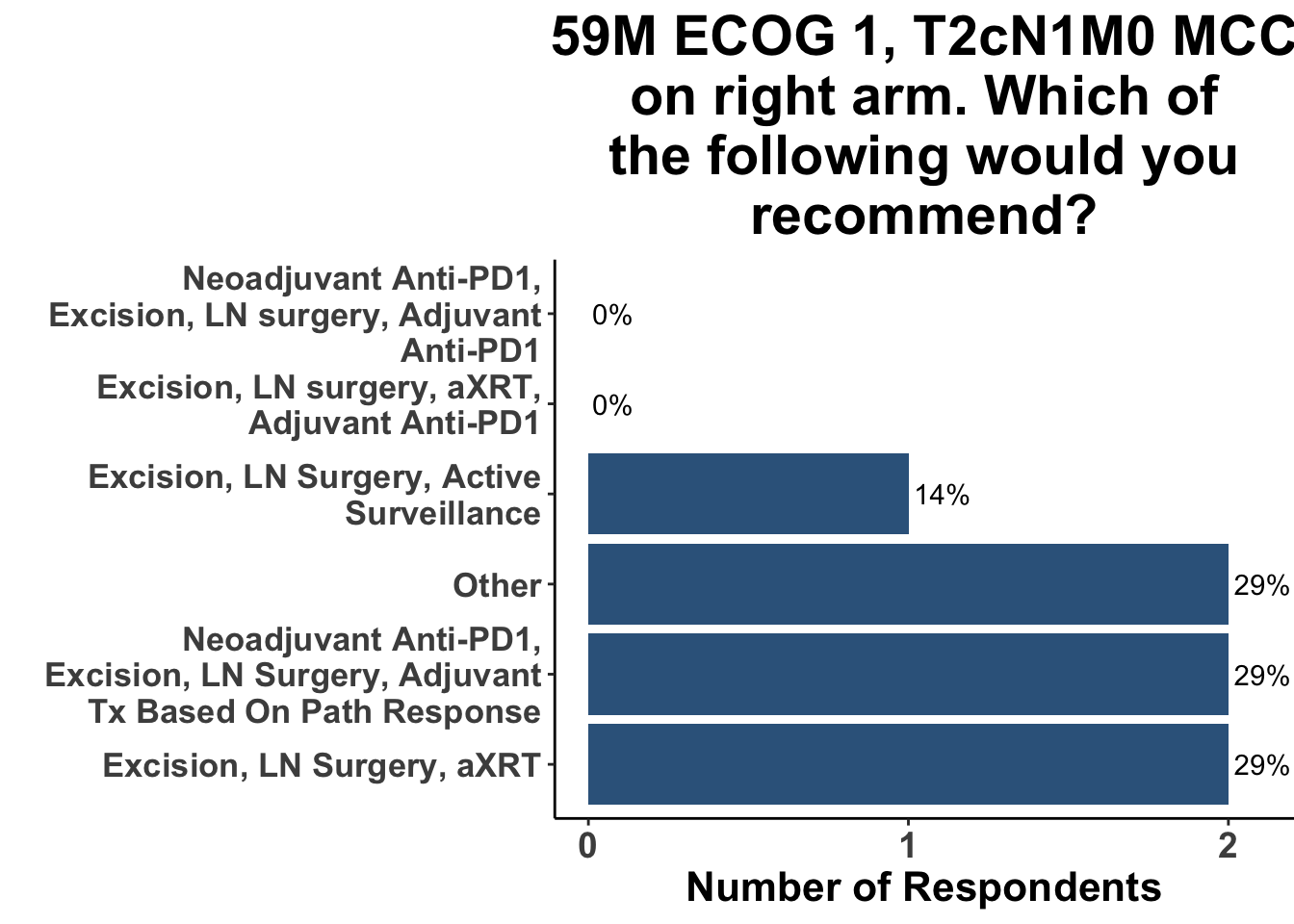
Given these strengths and weakness, what are the clinical implications of this study? When queried as to whether or not this paper would change their practice, there was a rare uniform consensus amongst the August 4th SoCO respondents: not a single clinician responded in the affirmative (Figure 4). To highlight this, when presented with a case of clinical stage III MCC, none of the providers selected a treatment plan commensurate with the experimental group in ADMEC-O (Figure 5).
Despite the promise of adjuvant ICI for patients with high-risk, resectable MCC, identifying the appropriate context for implementation, remains a challenge. For nearly a decade, immuno-oncolgists have wrestled with the dilemma of whether treating in the postoperative setting with anti-PD1/PD-L1 actually improves overall survival compared to waiting and treating patients at first evidence of macroscopic recurrence. Chemotherapists have long-held the view that treating in the fully-resected setting, when only microscopic disease remains, improves the likelihood of cure by decreasing the population of cells with intrinsic resistance. Whether this phenomenon holds in the setting of the immune checkpoint blockade remains unknown. What is known, however, is that by treating patients in the adjuvant setting, providers will be subjecting a subset of patients who are cured by surgery and/or radiation to unnecessary immune related adverse events. And until we know with a high-degree of confidence that treating in the postoperative period improves overall survival compared with the early macroscropic recurrent setting, many providers are persuaded by the Hippocratic oath of “first do no harm”. This is especially true of patients with early stage disease when the prognosis is favorable.
Further complicating the decision about adjuvant-only immunotherapy is emerging data suggesting superior outcomes with pre-operative ICI administration. A recent study of HRR melanoma patients reported that peri-operative anti-PD1 therapy, with three dose of pre-surgical pembrolizumab followed by 15 doses of post-operative pembrolizumab, was associated with improved event-free survival compared with post-operative only anti-PD1.26 These data allude to the importance of having macroscopic tumor antigens in situ to more effectively prime tumor-specific T cells compared with administering immune checkpoint inhibitors after resection. It is unknown if these results will translate to Merkel cell carcinoma. However, two doses of neoadjuvant nivolumab was associated with a major pathological response in 61.5% (16/26) of study subjects with resectable stage II-IV MCC in CheckMate-358.11 As a result, some providers have already incorporated this approach into the management of select patients with HRR disease (Figure 5). Therefore, this may limit the use of adjuvant-only ICI for HRR MCC.
And finally, the implementation of adjuvant immunotherapy from Merkel cell carcinoma will, in large part, be influenced by regulators. Given the exploratory nature of ADMEC-O it is unlikely that nivolumab will be granted a labeling indication for resected MCC. Since many payers incorporate FDA labeling in decisions about reimbursement, lack of a labeled indication will also limit its overall implementation in the clinic.
Conclusion
The ADMEC-O trial is an important contribution to the Merkel cell carcinoma field. The interim results from this well-conducted, phase 2 randomized study, suggest that post-operative ICI therapy connotes a clinically relevant reduction in recurrence for patients with MCC. The challenges experienced by the investigators highlights the need for accurate outcomes data so that prospective studies can be designed with the statistical power to address important clinical questions such as the role of post-operative immune checkpoint inhibitors. We eagerly await the final study results from this trial and from the other ongoing adjuvant trials in this space to help clarify the role of post-operative immunotherapy for HRR MCC.
Materials and Methods
This Perspectives on the Science piece was published using Quarto®. The survey was conducted using REDCap®.27 The figures depicting the survey data were created using R (version 4.0.0) and the tidyverse suite of packages,28 including ggplot2.29 The image on the “Perspectives on the Science” page was created by the authors (DMM) using the rosemary package.30 The figures depicting FDA approved therapies in skin cancer were created using the skincancerRx package.31
Bibliography
Appendix
Citation
@article{m. miller2023,
author = {M. Miller, David and Strong, Jennifer and S. Emerick, Kevin
and Gupta, Sameer and W. Silk, Ann and Brownell, Isaac},
publisher = {Society of Cutaneous Oncology},
title = {Adjuvant {anti-PD-1} for {Merkel} {Cell} {Carcinoma:} {Ready}
for the {Clinic?}},
journal = {Journal of Cutaneous Oncology},
volume = {1},
number = {2},
date = {2023-09-11},
url = {https://journalofcutaneousoncology.io/perspectives/vol_1_issue_2/adjuvant_nivolumab_for_mcc/},
doi = {10.59449/joco.2023.09.11},
issn = {2837-1933},
langid = {en}
}