Acid-Base Pearls Part 1
The Strong Ion Approach to Acid-Base Disorders
Acid-Base issues commonly seen on inpatient medicine
TABLE OF CONTENTS
The objective of this lecture is to provide a few clinical pearls on how to approach Acid-Base issues commonly seen on inpatient medicine. There is a slide component, which is used for didactic purposes, as well as a monograph section for background (see below). In part 1, we will introduce a methodology to approach clinical scenarios where Acid-Base Disturbances are key elements.
There are many excellent resources on this subject and the principles found in this lecture are an amalgam of several superb papers and monographs. Although I learned a great deal about Acid-Base physiology in Medical School and Residency, the EMCRIT podcasts by Scott D Weingart, MD were amongst some of the most valuable in my education. Please check them out.
Other resources include:
- Clinical review: Acid–base abnormalities in the intensive care unit by Lewis Kaplan and Spiros Frangos
- Physiological Approach to Assessment of Acid–Base Disturbances by Kenrick Berend, M.D., Ph.D., Aiko P.J. de Vries, M.D., Ph.D., and Rijk O.B. Gans, M.D., Ph.D
- Why Is Saline So Acidic (and Does It Really Matter?) by Benjamin AJ Reddi
- Integration of Acid–Base and Electrolyte Disorders by Julian Seifter MD
- History and Physical Exam
- Determination of the Primary Acid-Base Disorder and Secondary Response
- Evaluation of the Metabolic Component of the Acid-Base Disorder
- Evaluation for the presence of Mixed Metabolic Acid-Base Disturbances
- Consideration of the Serum (or Plasma) Osmolal Gap
- Evaluation of the Respiratory Component of an Acid-Base Disorder
- Interpretation of Acid-Base Disorders in the Clinical Context
The Range of pH that is compatible with life is 6.8 to 7.8 (a hydrogen ion concentration of 160 to 16 nanomoles per liter). Whether or not Acidosis or Alkalosis actually affects the body negatively has not been firmly established. Probably not between 6.8-7.8.
Rather, it is more likely that that the underlying problem that results in the acid/base distrubance (e.g. toxic substances, or hypoperfusion) causes the pathology, not the pH itself.
For example, does a hyperchloremic acidosis actually negatively affects one’s health? Unclear.
Does low pH decrease cardiac or catecholamine function? Still unclear.
PAO2 (Alveolar (calculated)) – PaO2 (the ABG O2)
A-a O2 Gradient = [ (FiO2) × O2 Pressure) - (PaCO2/0.8) ] – PaO2 from ABG
Normal A-a O2 Gradient Estimate = (Age/4) + 4
PAO2On Room Air, the PAO2 simplifies to:
150 - PCO2 x 1.25
(Given that Room Air is about 21% O2, the PAO2 = 0.21(760-47) ~ 150)
A-a O2 Gradient = (150 - PCO2 x 1.25) - PaO2
The Base Deficit (or Base Excess) is the amount of base or acid needed to bring the sample back to a pH of 7.4, after normalizing for a pCO2 of 40.
Thankfully, the Blood Gas analyzer performs an analsys by which it gets rid of any respiratory component that is contributing to the acid/base status. Thus, the Base Deficit (or Excess) will tell you if the patient has a metabolic acidosis or alkalosis.
For example:
- Base Excess of -6 (aka a Base Deficit of 6)
- What this means is that you’d have to add 6 mmol/l of base to get to a pH of 7.4
- i.e. the patient is acidotic
- What this means is that you’d have to add 6 mmol/l of base to get to a pH of 7.4
- Base Excess of 4
- i.e you’d have to add 4 mmol/l of acid to get to a pH of 7.4
- i.e. the patient is alkalotic
- i.e. the patient is alkalotic
- i.e you’d have to add 4 mmol/l of acid to get to a pH of 7.4
- Normal Range
- Normal -2 to +2
- If it is outside of this range it means there is an imbalance between Cations and Anions
- Normal -2 to +2
- This step-by-step method is adopted from Scott Weingart’s EMCRIT ACID-BASE SHEET, which is excellent!
- Blood Gas
- An Artertial or Venous Blood Gas will do, but VBGs are much easier to get, as a a phlebotomist can draw a VBG, but at all the hospitals I’ve worked at, MDs are the one’s (with a few exceptions) who must acquire an ABG
- Lactate
- Albumin
- this is a concentration, so getting one as proximal to the blood gas as possible is important as this can fluctuate
- this is a concentration, so getting one as proximal to the blood gas as possible is important as this can fluctuate
- Acetone
- Complete Metabolic Panel
- Urine Lytes (Na+, K+, Cl-)
- Urine pH
- If the blood pH is <7.35, the patient is acidotic
- At this point we don’t know if it’s metabolic, respiratory or mixed, but their primary problem is acidosis
- If the blood pH is >7.45 the patient is alkalotic
- Again, we don’t know if it’s metabolic, respiratory or mixed, but their primary problem is alkalosis
- Again, we don’t know if it’s metabolic, respiratory or mixed, but their primary problem is alkalosis
- If the blood gas C02 is > 45 they have a respiratory acidosis
- If the blood gas C02 is < 35 they have a respiratory alkalosis
- A strong ion is a salt that completes dissociates when put into solution
- e.g. NaCL -> Na+ + Cl-
- e.g. NaCL -> Na+ + Cl-
- Examples of strong ions include: Na+ K+ Ca2+ Mg2+ Cl-
- The difference between your strong ions is a major determinant of Acid/Base status
SodiumandChlorideare the major strong ions that determine acid/base (see figure below)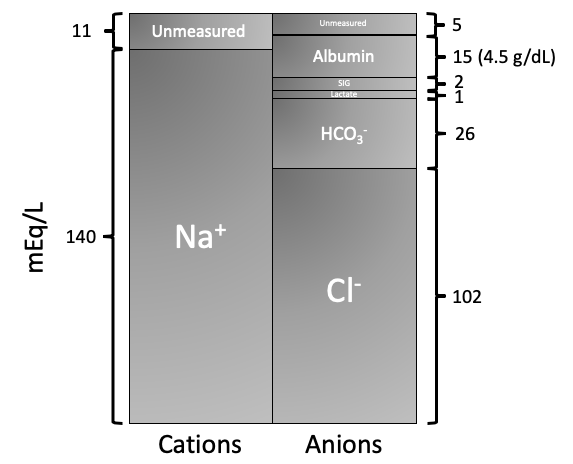
- To maintain electroneutrality, the body must either lose a negative charge or gain a positive charge
- The Strong Ion Difference that has clinical utility is the [Na+] - [Cl-]
- Thus, SID = [Na] - [Cl]
- Under normal conditions, this = 38
- Under normal conditions, this = 38
- Thus, SID = [Na] - [Cl]
- Thus, 38 is a good determinant of Acid/Base status
- If the SID is less than 38 -> think acidosis
- You can think of this as a relative increase in chloride
- This is often referred to as a “SID Acidosis”
- You can think of this as a relative increase in chloride
- If the SID is greater than 38 -> think alkalosis
- you can think of this as a relative increase in sodium
- This is often referred to as a “SID Alkalosis”
- If the SID is less than 38 -> think acidosis
- The three most common causes of a “SID Acidosis” are:
- IV Fluids
- Rental Tubular Acidoses
- Diarrhea
- IV Fluids
- Any fluid that has a SID of < 24 can cause an acidosis
- 2 liters of NS (which has a SID of 0) in <24 hours is enough to cause acidosis
- This is fascinating topic and worthy of a separate monograph, but in the meantime if you want more reading check out Scott Weingart’s post
- 2 liters of NS (which has a SID of 0) in <24 hours is enough to cause acidosis
- To work a suspected RTA up further, evaluate your Urine Studies
- Calculate a Urine Anion Gap (Urine Na+ + K+ – Cl-)
- If the UAG is negative, consider other causes as unlikely to be an RTA
- The UAG is a proxy for the ability of the kidney to secrete acid because it is hard to detect ammonium
- Chloride is excreted proportionally to NH4+ to maintain electroneutrality
- Thus, if the kidney can’t excrete acid i.e. in an RTA, the urine Chloride will be low, and the UAG will be positive
- The UAG is a proxy for the ability of the kidney to secrete acid because it is hard to detect ammonium
- If UAG is negative, then the kidney is secreting NH4+ and Cl- and you don’t have an RTA
- UAG is NOT useful in patients with proximal RTA
- If the UAG is negative, consider other causes as unlikely to be an RTA
- Assess
Urine pH- Type I (Distal RTA) ‐ Urine pH > 5.55
- causes include: auto-immune, sicklers, cirrhosis, idiopathic
- Type II (Proximal RTA) ‐ Urine pH < 5.55
- causes: myeloma, Wilson’s, Vit D deficiency, heavy metals
- Type IV (Distal RTA) ‐ Hyperkalemic, Urine pH < 5.5
- Aldosterone deficiency, diabetes
- Type I (Distal RTA) ‐ Urine pH > 5.55
- Calculate a Urine Anion Gap (Urine Na+ + K+ – Cl-)
- The loss of bicarbonate in the stool leads to a SID acidosis
- To work this up, look at the UCl
- Urine chloride <15 means that the person may have a saline-responsive alkalosis (though this also will depend on their volume status)
- UCl >15 means unlikely to have saline-responsive alkalosis
- UCl >15 means unlikely to have saline-responsive alkalosis
- Urine chloride <15 means that the person may have a saline-responsive alkalosis (though this also will depend on their volume status)
- Cyanide, Carbon Monoxide, Metformin, Didanosine,(Stavudine, Zidovudine, Linezolid, Strychnine, Emtriva, Rotenone (Fish Poison), NaAzide (Lab Workers), Apap (if Liver Fx), Phospine (rodenticide), NaMonofluoroacetate (Coyote Poison‐Give Etoh as antidote), Inh (if patient seizes), Hemlock, Depakote, Hydrogen Sulfide, Nitroprusside (If cyanide toxic), Ricin & Castor Beans, Propofol, Linezolid, Sympathomimetics (Cocaine, Methamphetamine), Jequirty peas (Abrus precatorius), Prunus Amygdalus Plants as well as Crab Tree Apple Seeds & Cassava (yucca)
- Of note, most of the toxins under SIG acidoses will also cause elevated lactate
- Rare causes
- Pyroglutamic acidemia (from taking tylenol in combination with severe sepsis, renal fx, or hepatic fx)
- Shoshin beri beri (from severe thiamine deficiency)
- Pyroglutamic acidemia (from taking tylenol in combination with severe sepsis, renal fx, or hepatic fx)
- Of note, the SIG is the Gap of Unmeasured Anions
- This is essentially the Anion Gap, but with the added information that we can calculate the lactate and we correct for albumin
SIG = (Base Deficit) + (SID – 38) + 2.5 (4.2 ‐ Albumin (g/dL)) – Lactate - This can also be thought of as the corrected base deficit, or put a minus sign in front and it is the corrected base excess
- Could also be thought of as the Acid Excess (with Acid Excess = Base Deficit)
Acid Excess = SIG + (38 – Patient’s SID) + 2.5(Pt’s Albumin – 4.2) + Lactate - In other words, the acid excess seen on the VBG must be explained somehow
- This is probably the most logical way of remembering the formula
- The Excess Acid (if there is any), could be explained completely by:
- Lactate
- Excess albumin (b/c albumin is a weak acid), though this never really happens
- SID acidosis (aka non-anion gap acidosis)
- Strong Ion Gap Acidosis (Uremia, DKA, AKA, Tox, D-lactic Acidosis)
- Lactate
Metabolic Acidosis present
- Uremia, DKA, AKA (Alcoholic KetoAcidosis)
- Tox
- ASA, ethylene glycol, methanol, propylene glycol (ativan, valium, dilantin infusions), iron, INH, & paraldehyde
- D-Lactic Acidosis
- from short gut/blind loop
- this will not show on lactate assay
- from short gut/blind loop
- Hypercalcemia, Hypermagnesemia, Hyperkalemia, Immunoglobulins, Bromide, Nitrates, Lithium Overdose
- If the primary disturbance is respiratory and you feel it is chronic, you can calculate the expected metabolic compensation
- Expected Δ BE (or expected ↓ of SID) = 0.4 x (Chronic Change in CO2)
- If the primary problem is metabolic acidosis
- Expected ↓ CO2 = Base Deficit
- If the primary problem is metabolic alkalosis
- Expected ↑ CO2 = 0.6 x Base Excess
- Formula to correct PaCO2 in a COPD Patient
- 0.08 decrease in pH = for every 10 mmHg increase in PaCO2 acutely
- If the SIG is elevated and the above work up has not yielded a diagnosis, the next step is to look for an
Osmolar Gap- Normal Osmolality is 270-290 mOsm/Kg H2O
Osm Gap = Measured Osmal – (2 Na + Gluc/18 + BUN/2.8 + ETOH/3.7)
- Normal Osmolality is 270-290 mOsm/Kg H2O
- An
Osmomar Gap >10is considered “Positive”- Causes of Elevated Osm Gap:
- Methanol, Ethylene glycol (found in automotive anti-freeze), mannitol, isopropanol (isopropyl alcohol), propylene glycol (found in Lorazepam, Valium, Phenobarb, Phenytoin infusions), lithium
- Methanol, Ethylene glycol (found in automotive anti-freeze), mannitol, isopropanol (isopropyl alcohol), propylene glycol (found in Lorazepam, Valium, Phenobarb, Phenytoin infusions), lithium
- Causes of Elevated Osm Gap:
- Distinguishing between toxic alcohols
- Methanol and Ethylene Glycol cause BOTH increase in AG and Osm Gap
- Isopropyl Alcohol causes an increase in Osm Gap, but NOT AG
- Methanol and Ethylene Glycol cause BOTH increase in AG and Osm Gap
- If Osm Gap is >50
- The etiology is almost certainly a toxic alcohol
- The etiology is almost certainly a toxic alcohol
- Assay for Toxic Alcohols (volatile alcohols)
- Liquid/Gas Chromatography
- Tx for Ethylene Glycol Poisoning
- Dialysis
- Bicarb
- Fomepizole (Alcohol Dehydrogenase Inhibitor)
- Give until level of EG undetectable
- Dialysis