Impact of an Evolving Regulatory Landscape on Skin Cancer Drug Development in the U.S
Article and Authorship Details
Article type: This is the pre-print version. Please see the peer-reviewed version here.
Authors: David M. Miller1* MD PhD, Sophia Z. Shalhout1 PhD, Denise Casey3 MD, Lola Fashoyin-Aje2 MD, Steven Lemery2 MD, Marc R. Theoret2 MD, Richard Pazdur2 MD
1Department of Medicine, Division of Hematology/Oncology and the Department of Dermatology, Massachusetts General Hospital, Boston, MA 2The Food and Drug Administration, Silver Spring, Maryland. 3DataRevive LLC, Rockville, MD 20850
*Corresponding author: David M. Miller MD PhD
Massachusetts General Hospital
Email: dmiller4@mgh.harvard.edu
Funding sources: None
Conflicts of interest: David M. Miller has received honoraria for work on advisory boards for Pfizer Inc., Merck Sharpe & Dohme, Sanofi Genzyme, Regeneron, EMD Serono, and Checkpoint Therapeutics. This article reflects the views of the authors and should not be construed to represent the views or policies of the FDA.
Manuscript word count: 2493
Abstract word count: 149
References: 12
Figures: 8
Tables: 2
Keywords: regulatory medicine, cutaneous oncology, melanoma, Merkel cell carcinoma, squamous cell carcinoma
Abbreviations: 2D Measurement: Sum of two dimensional tumor measurements, ACTG: AIDS Clinical Trials Group Oncology Committee of the National Institute of Allergy and Infectious Diseases criteria, AK: actinic keratosis, BCC: basal cell carcinoma, CCR: complete clearance rate, CTCL: cutaneous T-cell lymphoma, DESI: Drug Efficacy Study Implementation, DFSP: dermatofibrosarcoma protuberans, FDA: US Food and Drug administration, FDAAA: Food and Drug Administration Amendments Act, FDAMA: Food and Drug Administration Modernization Act, FDARA: Food and Drug Administration Reauthorization Act, FDASIA: Food and Drug Administration Safety and Innovation Act, GRS: global response criteria, HDAC: histone deacetylase, KS: Kaposi’s sarcoma, laBCC: locally advanced basal cell carcinoma, laCSCC: locally advanced cutaneous squamous cell carcinoma, lb95%CI: lower bound 95% confidence interval, mBCC: metastatic basal cell carcinoma, MANUF (CMC): Chemistry, Manufacturing and Controls, MCC: Merkel cell carcinoma, mCSCC: metastatic cutaneous squamous cell carcinoma, mRECIST: modified RECIST, mSWAT: modified SWAT, ORR: overall response rate, OS: overall survival, PFS: progression-free survival, PHBPA: Public Health and Bioterrorism Preparedness Act, PK: pharmacokinetic analysis, RECIST: Response Evaluation Criteria in Solid Tumors, r/mCSCC: recurrent or metastatic cutaneous squamous cell carcinoma, rCTCL: refractory cutaneous T-cell lymphoma, RFS: relapse-free survival, SCC: squamous cell carcinoma, SWAT: severity-weighted assessment tool, u/mMelanoma: unresectable or metastatic melanoma, U.S.: United States, WHO: world health organization; WSSI: weighted skin severity index.ABSTRACT
Background: There has been a rapid proliferation of FDA-approved medications with labeled indications for skin cancer over the last decade, with particular growth over the last 5 years.
Objective: We aimed to evaluate the impact of an evolving U.S. regulatory framework on drug development programs to better understand current trends and regulatory considerations when adjudicating drug approvals for patients with skin cancer.
Methods: We reviewed publicly-available regulatory documents of all systemic medications with a labeled indication for skin cancer.
Results: We identified 130 FDA approvals that resulted in a unique indication, usage, formulation or dosage change in skin cancer since 1949.
Limitations: Publicly available data from the mid-to-late 20th century is limited.
Conclusions: The therapeutic landscape in skin cancer has changed greatly since the first approval in 1949. In concert, regulatory medicine has also evolved over the last 70 years with the aim of ensuring safe and effective medicines for a diverse array of patients.
INTRODUCTION
Skin cancer is a heterogeneous group of malignancies that result from neoplasia of keratinocytes, melanocytes, adnexal structures, nervous tissue, stromal cells and effectors of the innate and adaptive immune system. In aggregate, new cases of skin cancer outnumber all other forms of cancer combined, with keratinocyte carcinoma alone accounting for 3-4 million cases in the US every year1. In keeping with the diverse mechanisms that give rise to cutaneous malignancy, there are a heterogeneous group of drug therapies used to treat skin cancer, which includes cytotoxic agents, targeted therapies, and immunotherapies (Table 1). Analogous to the changing therapeutic landscape in cutaneous oncology, the field of regulatory medicine has also evolved substantially over the last century.
To understand how current trends and regulatory approaches to adjudicating drug approvals for skin cancer are influenced, it is useful to also recognize the evolution of regulatory medicine in general. For example, the FDA’s modern regulatory function commenced with the Pure Food and Drugs Act of 1906, which prohibited the manufacturing and distribution of misbranded products. Galvanized in large part by the Elixir of Sulfanilamide disaster, Congress passed the Federal Food, Drug, and Cosmetic (FD&C) Act in 1938. The FD&C Act contained provisions requiring new drugs to be shown safe before marketing. In 1962, the FD&C statute was amended to require proof of efficacy in addition to safety prior to granting marketing approval (Figure 1).
Figure 1. Landscape of Regulatory Medicine and Cutaneous Oncology
Figure 1. Landscape of Regulatory Medicine and Cutaneous Oncology
Key landmark acts responsible for shaping and influencing trends in regulatory medicine are overlaid among the FDA Approvals specific to cutaneous oncology. Briefly, the Pure Food and Drugs Act of 1906 prohibited “the manufacture, sale, or transportation of adulterated or misbranded or poisonous or deleterious foods, drugs or medicines, and liquors”2. In 1937, more than 100 people died secondary to ingestion of diethylene glycol contained in the Elixir Sulfanilamide3. Subsequently, Congress passed the Federal Food, Drug, and Cosmetic Act (FD&C) in 1938, which required new drugs to be safe prior to marketing. In 1962, Congress passed amendments to the FD&C, commonly known as the Kefauver-Harris Amendments, which required that sponsors demonstrate scientific evidence of the effect of the purported labeling claim, not just safety. The statutory requirement remains that to obtain marketing approval, sponsors must provide substantial evidence of the drug’s safety and effectiveness for its intended use. Responding to complaints about delays in drug approvals, Congress passed the Prescription Drug User Fee Act (PDUFA) in 1992, which permitted the FDA to collect fees from sponsors in order to provide more efficient and timely reviews of new drug and biologic applications. PDFUA must be reauthorized every five years. It was renewed in 1997 with FDAMA (Food and Drug Administration Modernization Act), 2002 as part of the Public Health and Bioterrorism Preparedness Act (PHBPA), 2007 as FDAAA (Food and Drug Administration Amendments Act), 2012 with FDASIA (Food and Drug Administration Safety and Innovation Act) and in 2017 as part of FDARA (Food and Drug Administration Reauthorization Act). Each reauthorization incorporates initiative and programs to continue optimizing the regulatory role of the FDA. The 21st Century Cures Act was passed by the 114th Congress with the goal to accelerate the discovery and development of new therapies. Subtitle C, section 3022 called for a program to evaluate the potential use of real world evidence to help support regulatory decisions. Abbreviations - FDAAA: Food and Drug Administration Amendments Act; FDAMA: Food and Drug Administration Modernization Act; FDARA: Food and Drug Administration Reauthorization Act; FDASIA: Food and Drug Administration Safety and Innovation Act; PHBPA: Public Health and Bioterrorism Preparedness Act.
In this article, we report an examination of the totality of drug approvals for skin cancer with a specific focus on the evolving regulatory landscape as it pertains to the assessment of clinical benefit. We highlight changes in regulatory considerations regarding trial design, efficacy endpoints, and the overall benefit:risk assessment as they relate to labeling claims for use in patients with skin cancer. Knowledge of the regulatory framework allows physicians, investigators, industry and regulators to promote continued innovation in the regulatory sciences and, consequently, increases efficiency in development of safe and effective medications.
METHODS
Overview
To evaluate the evidence used to support labeled claims in skin cancer we reviewed FDA New Drug Application (NDA) or Biological License Application (BLA) reviews, and the US product labels, that are indexed on the FDA website (https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm) from 1949 to February 09, 2021.
Data
In addition to the product labels and NDA and BLA reviews, data were also obtained from OpenFDA (https://download.open.fda.gov/drug/drugsfda/drug-drugsfda-0001-of-0001.json.zip).
Disease Selection
We chose the following eight skin neoplasia: actinic keratosis, basal cell carcinoma, cutaneous T-cell lymphoma, dermatofibrosarcoma protuberans, kaposi’s sarcoma, melanoma, Merkel cell carcinoma, and cutaneous squamous cell carcinoma. These were selected for our analysis because each category has at least one FDA-approved therapy. Actinic keratosis, although not an overt malignancy, but rather a pre-malignant condition, was included because of its importance in the management of skin cancer and ubiquitous presence in dermatological practice. In general, drugs for actinic keratosis and local treatments for BCC and SCC are reviewed outside of the oncology review Divisions within the FDA.
Therapeutic Inclusion Criteria
Therapies that have a labeled indication for skin neoplasia (as defined above) were selected. Only labeling modifications of NDAs or BLAs were selected. If a molecular entity had an abbreviated new drug application (ANDA) associated - e.g., a generic form of the therapy was approved), the ANDA was not included in the analysis, as these generally not required to include clinical data to establish safety and efficacy.
Endpoint Selection
Primary endpoints were included in our study as they are the principal analytical criteria for labeling modification. Secondary endpoints are outcomes that are related to the primary question and are often used to support the conclusions derived from the primary question. They are, however, often not sufficient to support a labeled claim. Therefore, we did not include them in this primary analysis. We still emphasize the importance of secondary endpoints, as they can play an integral role in the evaluation of an agent’s efficacy.
Applications Selected for “Patients Per Pivotal Trial” and “Trial Design” Analysis
For our analysis of the number of patients used in pivotal trials as well as the trial design, we chose application submissions that led to either initial approval of a product (e.g., a Type 1, 3 or 5 submission) or a supplemental approval that resulted in a new indication or usage. Actions that were based solely on pharmacokinetic analysis (e.g., NDA022067, BLA125554, NDA019157, BLA125514, BLA125377) were excluded.
Missing Data
Data on investigational agents approved prior to the Kefauver-Harris Amendments were not obtainable (n = 23). Therefore, efficacy data and trial design on these drugs, known as DESI (Drug Efficacy Study Implementation) drugs are limited. Furthermore, certain data regarding trial design, type of submission and subject enrollment for therapies approved in the mid-to-late 20th century were also not available. We understand that this is a caveat of this figure. Missing data can be appreciated for all actions by looking at Table 1.
Data Visualizations
Figures were generated with the package ‘ggplot2’, using the R programming language, version 4.0.0 (R Foundation for Statistical Computing).
Additional Clarifications
In regards to NDA 022483/S-003. The initial application for Zyclara included trials studying both 3.75% and the 2.5% dosage forms. The sponsor submitted for an initial NDA for the 3.75% cream. S-003 was a supplemental approval for the 2.5% dosage form. The initial NDA (NDA 022483) included 319 patients. The updated label for S-003 includes data on 479 patients. The drug development program compared both the 3.75% (n = 160) and the 2.5% (n = 160) dosage forms against subjects who received a vehicle control (n = 159). Since there are two different NDA numbers and dates of approval, we have used 319 and 160 respectively so that the total number patients in the studies would be 479, which is reflected in the label.
RESULTS
FDA Approvals in Skin Cancer
Since 1949 there have been 76 drugs approved with an indication or usage for skin cancer. We identified 1518 modifications to the marketing labels of those therapies, including approvals for use, as well as labeling updates (Table 2). To gain further insight into regulatory decisions governing approval, we focused our analysis on the 130 FDA actions that resulted in the approval of a product for a new or revised indication or usage, a formulation change, or dosage change in eight distinct types of cutaneous neoplasia with at least 1 FDA-approved agent at the time of our analysis (Figures 1 & 2).
Figure 2. FDA Approvals Per Decade
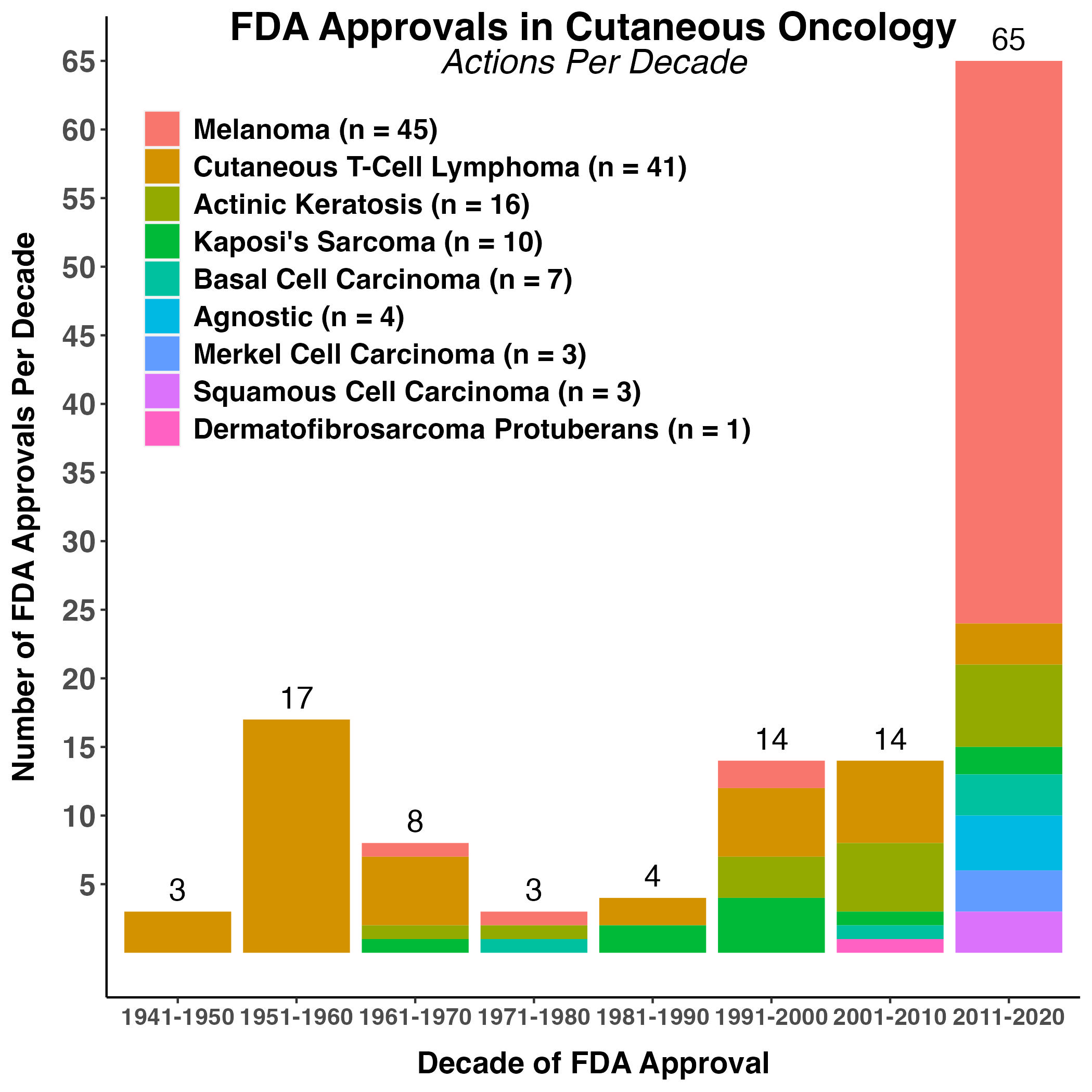
Figure 2. FDA Approvals Per Decade
The number of regulatory decisions relating to indications and usage, formulation changes, dosage and administration made in skin cancer drug labels per decade are shown, with color indicating the skin neoplasia corresponding to that action. Parenthetically in the legend is the total number of actions per disease from 1949-2021 by skin cancer type.
Among the 130 FDA approvals in our analysis, 66% occurred in two diseases: melanoma (n = 45) and cutaneous T-cell lymphoma (CTCL) (n = 41). There were 38 new molecular entity (NME) approvals and 63 approvals of efficacy supplements for skin cancer. This constituted 78% of the approvals over the last 70 years (Figure 3).
Figure 3. Type of Labeling Modification
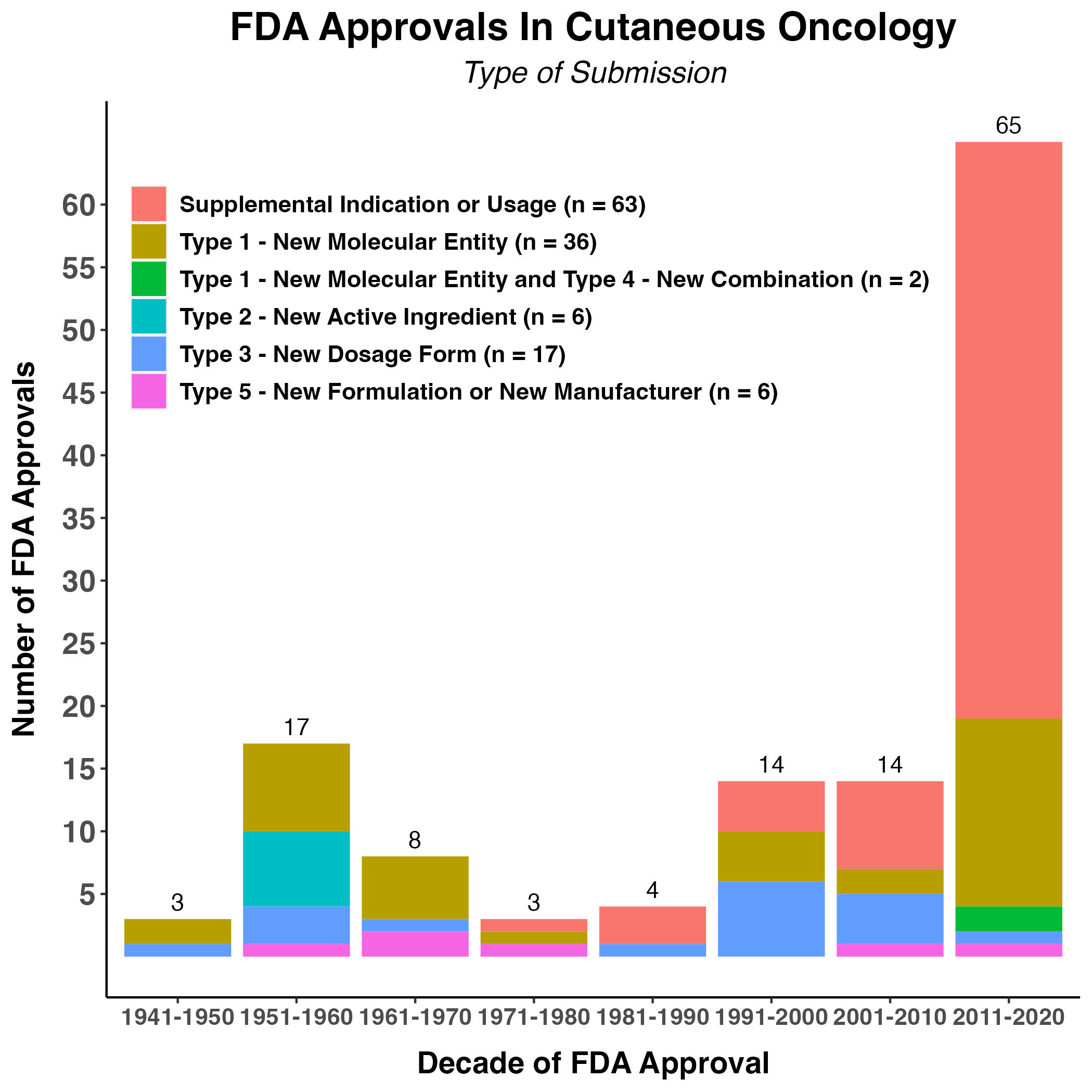
Figure 3. Type of Labeling Modification
This figure depicts the different types of modifications made in skin cancer product labels across the 8 categories of skin neoplasia that have approved therapies, as well as approvals for therapies without specific skin cancer indications (aka “agnostic”), but with relevant use in skin cancer populations. Parenthetically in the legend is the total number of validated labeling modifications corresponding to that type from 1949-2021.
The rate of FDA approvals for skin cancers has changed prominently over time (Figure 2). There were 3 FDA approvals in cutaneous oncology during the 1940s. In the 1950s there were an average of 1.7 actions per year, followed by 0.3 to 1.4 actions annually up until 2010. In the final full decade of our analysis (2011 – 2020), FDA approvals per year increased substantially to 6.5. Indeed, 62% (81/130) of the approvals in our analysis occurred in the last two decades, and 73% (95/130) since the enactment of the first Prescription Drug User Fee Act (PDUFA) on October 29, 1992. The average rate of approvals has steadily increased over the last four rounds of PDUFA re-authorization (Figure 1).
Primary Endpoints Used in FDA Approvals
Figure 4. Primary Endpoints Used in Regulatory Decisions for Skin Cancer Products
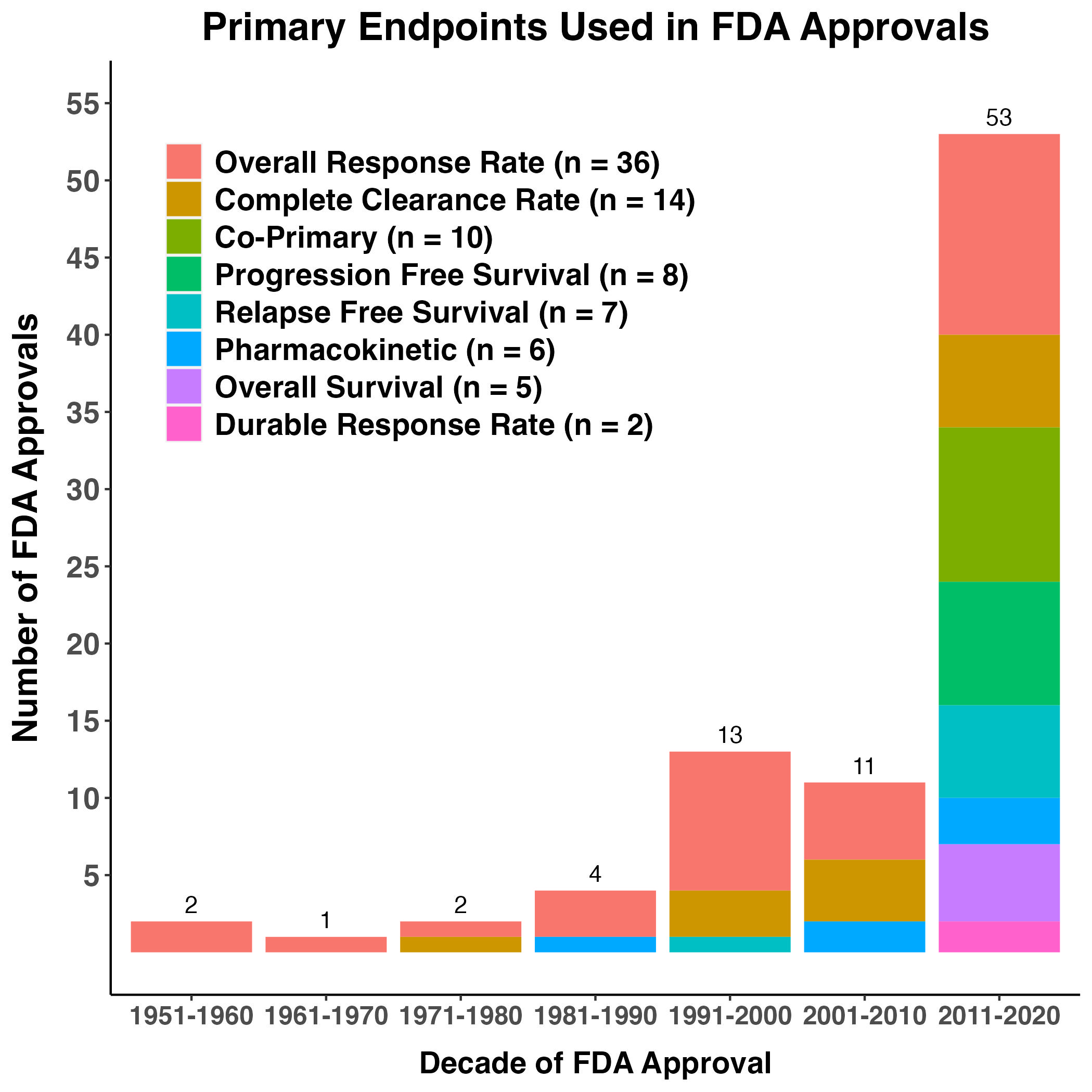
Figure 4. Primary Endpoints Used in Regulatory Decisions for Skin Cancer Products
Depicted is the cumulative number and type of primary endpoints used in pivotal trials for skin cancer by decade. Bars are filled with colors corresponding to the type of primary endpoint used in the pivotal trials for NDA or BLA submission from 1951-2020. Co-primary represents trials that used more than one primary endpoint in a pivotal trial. Parenthetically in the legend is the total number of times the specific endpoint was utilized since 1951. Abbreviations - CCR: complete clearance rate, ORR: overall response rate, OS: overall survival, PFS: progression-free survival, PK: pharmacokinetic analysis, RFS: relapse-free survival.
Of 89 reviewable labeling modifications, 64% (57/89) incorporated some form of tumor response claim (e.g., overall response or complete clearance rate). Overall survival, a gold-standard endpoint to measure direct clinical benefit of systemic therapies in oncology trials, was used in 10 applications (5 applications where it was the sole endpoint and 5 applications were it served as a co-primary endpoint), and has only been used in the most recent decade.
Approval Pathways Used in FDA Approvals
All FDA-approved products must meet statutory standards for safety and effectiveness. A regular approval is supported by endpoints that measure direct clinical benefit (e.g., improved survival, symptoms or functional impairments) in the intended use population. The Accelerated Approval (AA) pathway was established in 1992 for therapies used to treat serious and life-threatening conditions with an unmet medical need (21 CFR 314 Subpart H for drugs and 21 CFR 601 Subpart E for biologics). As amended by FDASIA, the FD&C Act provides FDA the authority to grant an AA based upon “determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments.”4 Since 1992, there have been 95 FDA approvals of marketing applications seeking new or updated skin cancer indications or usage, including 21 via the AA pathway. The vast majority of AAs occurred in the last 10 years (19/21), including 67% in the last five years (Figure 5).
Figure 5. Accelerated Approval Pathway
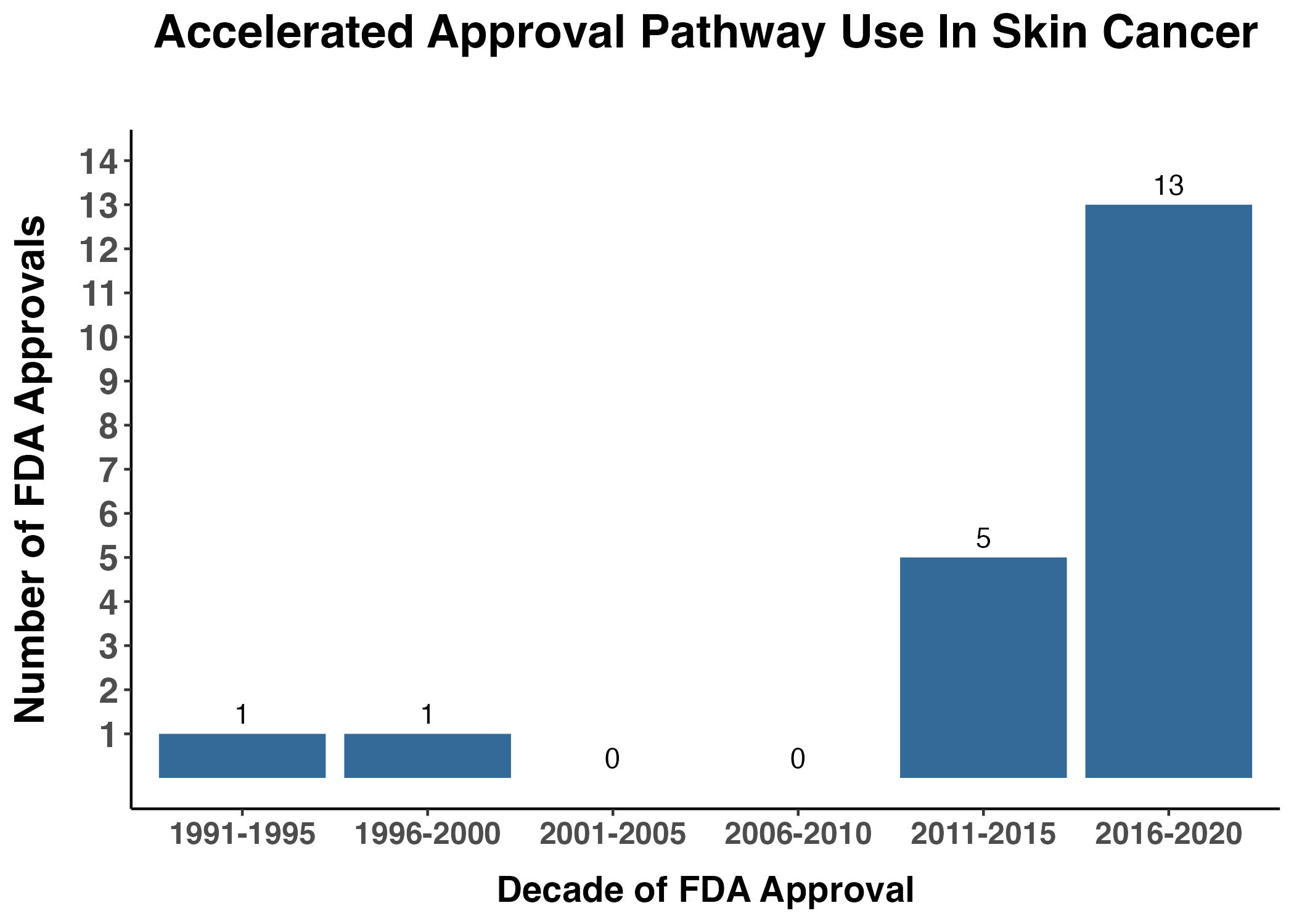
This graph depicts the cumulative number of applications specific for indications and usages in skin neoplasia that were approved via the accelerated pathway from 1991-2020.
Criteria Used in Assessment of Trial Endpoints
Consistent with the diversity in endpoints used to support labeling claims, a number of criteria have been used to evaluate trial endpoints, with Clinical Assessment (e.g., evaluation of alive/dead status) being the most commonly used (Figure 6). Since skin lesions are not always amenable to reliable radiographic evaluation, visual assessment and clinical photography have played essential roles in response assessment. Standardized photographs of baseline and treated skin lesions are recommended in scoring assessment tools in CTCL (e.g., mSWAT and GRS)5. These criteria were used in the trials supporting the approvals of vorinostat, brentuximab vedotin and mogamulizumab-kpkc in CTCL.6,7,8 A composite criteria of bi-dimensional measurements of tumor ulceration and standardized digital photography oftarget lesion(s) in patients with locally advanced BCC (laBCC) was used to support the 2012 NDA for vismodegib. Similarly, a protocol-specific modified RECIST was incorporated for assessing patients with laBCC in the 2015 NDA for sonidegib. This modification of RECIST utilizes multiple data sources including digital photography, magnetic resonance imaging, and pathology from tumor biopsies to develop a composite endpoint called “composite overall response”. Notably, although long used as a method of standardizing assessments of tumor response in skin cancer lesions, digital photographic evaluations conducted during recent pivotal trials served as part of the totality of data supporting FDA granting regular approvals to hedgehog inhibitors for advanced BCC and PD-1 monoclonal antibodies for advanced CSCC and laBCC.9,10,11,12 While ORR more commonly serves as an intermediate endpoint supporting AA in other cancer indications, the dramatic and treatment-related improvements in baseline disfiguring BCC and CSCC lesions observed in the photographic data included in the marketing applications, and the durability of these effects, was considered evidence of direct clinical benefit in these skin cancer populations.Figure 6. Criteria Used for Assessment of Primary Endpoint
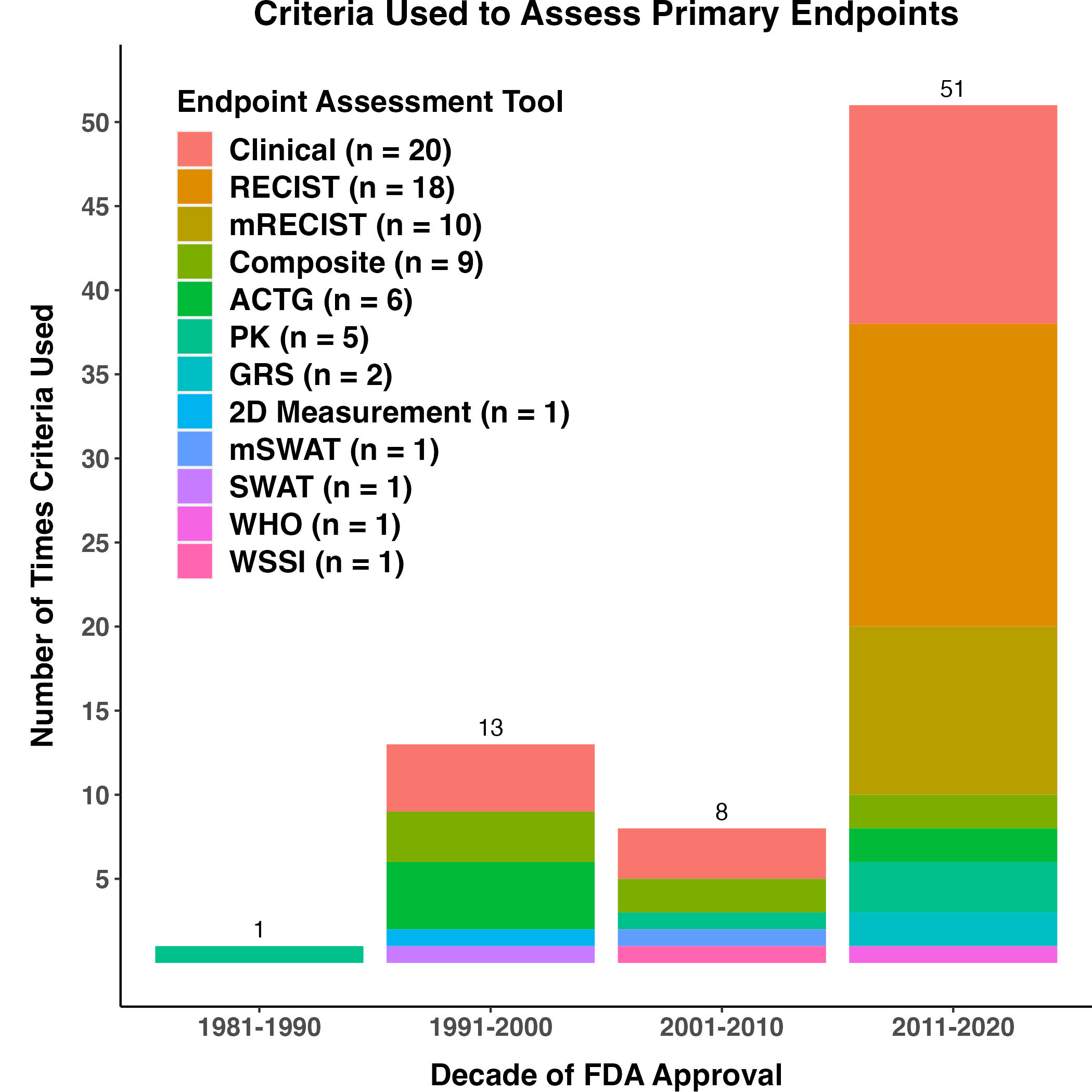
Figure 6. Criteria Used for Assessment of Primary Endpoint
Criteria used for primary endpoint assessments are shown by decade of FDA Approval from 1981-2020. Parenthetically in the legend is the total number of times that tool was used by the applications in this study since 1981. Abbreviations: 2D Measurement: Sum of two dimensional tumor measurements, ACTG: AIDS Clinical Trials Group Oncology Committee of the National Institute of Allergy and Infectious Diseases criteria (this group includes both ACTG and modified ACTG), CCR: complete clearance rate, Clinical: this connotes an investigator assessment without a specific tool, e.g., detection of a recurrent lesion in an adjuvant study or absence of a lesion for complete clearance rate assessment), Composite: composite endpoint, GRS: global response criteria, mRECIST: modified RECIST, mSWAT: modified SWAT, ORR: overall response rate, OS: overall survival, PK: pharmacokinetic analysis, PFS: progression-free survival, RECIST: Response Evaluation Criteria in Solid Tumors, RFS: relapse-free survival, SWAT: severity-weighted assessment tool, WHO: world health organization; WSSI: weighted skin severity index.
Patients Per Pivotal Trial
Figure 7. Subjects Enrolled in Pivotal Trials
Figure 7. Subjects Enrolled in Pivotal Trials
The number of patients in each pivotal trial is depicted as a red dot grouped by decade of FDA Approval from 1971-2020.
Efficacy Assessment in Non-Comparator Trials
Information regarding design of the pivotal trial was available for 79 approvals in our analysis. Of those, 24 labeling modifications were supported by placebo-controlled trials, 25 by trials of the study drug versus an active-comparator, and 30 by non-randomized studies. Single-arm trials often measure efficacy based on tumor response rate and duration of response since time-to-event endpoints (e.g., survival) are challenging to interpret in the absence of an internal control (whereas most tumors rarely shrink in the absence of therapy). Of the 30 labeling modifications supported by results of single-arm studies, the observed ORR ranged from 15.9-83.3%, with a median of 34% (Figure 8). ORR results are typically evaluated in the context of a pre-specified target overall response rate and a lower bound 95% confidence interval (lb95%CI) considered clinically relevant in the respective indication. We identified 15 labeling modifications in which the lb95%CI was pre-specified in the protocol. A lb95%CI that excluded response rates less than 15% was the most commonly used boundary (n = 5) in the statistical plans; the range was 5% (n=4) to 25% (n=2). The lb95%CI appears to be increasing in the protocols over time with 5% last used in 2006. Between 2016 and 2020 the minimum lb95%CI was 15%. Furthermore, the median lb95CI has increased from 5% in 1996-2000 to 17.5% in 2016-2020.
Figure 8. Efficacy Assessment in Non-Comparator Trials
Figure 8. Efficacy Assessment in Non-Comparator Trials
Depicted is the overall response rate (filled circles) in non-comparator trials with the corresponding 95% confidence interval (dotted segments) and the lb95%CI proposed by the sponsor to estimate a clinically meaningful response rate (“X”). This is conceptually similar to a null hypothesis for which an investigator would reject if the data were statistically persuasive. For actions, FDA may focus on the observed response rate and associated confidence interval rather than original statistical design of the protocol. Abbreviations. AIDS KS: AIDS-related kaposi sarcoma, AIDS KS (AA): AIDS-related kaposi sarcoma, trial approved with accelerated approval, HIV-pos KS: HIV-positive Kaposi Sarcoma, HIV-neg KS, HIV-negative Kaposi Sarcoma, laBCC: locally advanced basal cell carcinoma, laCSCC: locally advanced cutaneous squamous cell carcinoma, la/mMCC: locally advanced or metastatic Merkel cell carcinoma, lb95%CI: lower bound 95% confidence interval, mBCC: metastatic basal cell carcinoma, mCSCC: metastatic cutaneous squamous cell carcinoma, MSI-hi: microsatellite instability high, r/mCSCC: recurrent or metastatic cutaneous squamous cell carcinoma, rCTCL: refractory cutaneous T-cell lymphoma, TMB-hi: tumor mutation burden high, u/mDFSP: unresectable or metastatic dermatofibrosarcoma Protuberans, u/mMelanoma: unresectable or metastatic melanoma.
Discussion
The therapeutic landscape in skin cancer has changed greatly since the first approval in 1949. In concert, regulatory medicine has evolved over the last 70 years with the aim of ensuring access to safe and effective medicines for a diverse array of patients. While approvals for CTCL have occurred steadily over the last 70 years, therapeutic innovation in melanoma has been a more recent development, with 91% of actions occurring in the last ten years. Furthermore, in just the last five years, initial approvals occurred in Merkel cell carcinoma, cutaneous squamous cell carcinoma, and tissue agnostic indications with direct relevance to skin cancer.
The 1962 Statutory Effectiveness Standard FDCA Sec 505(b) put into law that a sponsor must provide “substantial evidence that the drug will have the effect it purports or is represented to have under the conditions of use prescribed, recommended, or suggested in the proposed labeling”. The primary basis for determining whether there is “substantial evidence” to support the claims of effectiveness for new drugs are reports of adequate and well-controlled investigations. Therefore, although there are statutes codified into law that provide guidance to the FDA, benefit-risk assessments must be interpreted through the contextual lens of the disease environment specific for any given labeled indication. Isolation of the purported effect may dictate that trials be designed with multiple arms, incorporating either a placebo or active-comparator control. Randomization is generally necessary for a reliable analysis of time-to-event endpoints in oncology trials (e.g. PFS or OS). Use of OS in clinical trials in cutaneous oncology has generally occurred in the past decade. This is likely context dependent, as improvements in OS have supported melanoma approvals which have most occurred over the prior decade. As melanoma is a systemic disease, approvals generally require data to support a systemic anti-tumor effect (and survival is a gold standard for such an effect).
The phrase “adequate and well-controlled studies” has commonly been interpreted as a requirement for external duplication through at least two independent trials. However, FDA may also rely on a single clinical trial to establish effectiveness in certain circumstances. In cutaneous oncology specifically, single-arm trials demonstrating a clinically relevant effect size on ORR that is durable have successfully supported marketing approvals of several new molecular entities and supplemental indications.
Depending on the context, ORR in cancer studies may be considered a surrogate or intermediate clinical endpoint that is “reasonably likely to predict clinical benefit”; therefore, an effect on ORR has the potential to support an AA with requirements to verify and describe benefit in subsequent post-marketing studies. It is noteworthy however, that for some skin cancer indications, there are multiple examples of regular approvals based on demonstration of durable response rates in the intended use population. As discussed above, for recent drug approvals for advanced BCC and CSCC, FDA evaluated the totality of data for these products in the context of patients having a life-threatening cancer as well as other serious disease burdens. Demonstration of durable ORRs that correlated with substantial reduction in size of disfiguring cutaneous tumors in highly visible areas, was considered direct evidence of clinical benefit and supported these regular approvals. Ultimately, the choice of endpoint in any specific development program will depend upon multiple factors including ability to accurately assess the endpoint, unmet need, rarity of the disease, and the relative efficacy of the drug.
Because cutaneous oncology is diverse with respect to goals of therapy, available therapies, unmet need, and prevalence of the different diseases, there are different considerations for each disease with respect to study designs including number of patients needed to demonstrate efficacy. For example, the smallest trial in this cohort, the 2006 supplemental NDA for imatinib in DFSP, consisted of 18 subjects. This application was supported by data from 12 subjects in a single-arm trial and 6 from case reports. Central to the regulatory decision was the fact that there were no available therapies approved for DFSP (an ultra-rare disease), the biology was well characterized, the safety profile of imatinib was well known, and the results were statistically persuasive and consistent with similar response rates in other approved conditions of use. For example, 83% (15/18) of the patients in the application had a response with 47% (7/15) of responders demonstrating a complete response.
For rare cutaneous malignancies, global drug development strategies may be needed and developers may use novel trial designs to assess the effects of a drug. Such designs may include basket or umbrella trials, trials with Bayesian designs, or trials that generate evidence for tissue agnostic approvals. As stated in Figure 1, one goal of the 21st Century Cures Act is to evaluate the use of “Real World Evidence” (RWE) to support regulatory decisions. For example, photographic evidence of clinical benefit, a unique feature of cutaneous oncology, could be incorporated into RWE.
Caveats of this study include the fact that data were not obtainable for every approved product. Additionally, although safety is a critical component of the benefit-risk evaluation, this study focused on the assessment of clinical benefit, with a scope limited primarily to efficacy.In summary, regulatory decisions regarding the indications and usage of anti-neoplastic agents occur in a dynamic environment. An improved understanding of the trends of regulatory medicine by dermatologists and oncologists may yield more effective clinical investigations for patients with skin cancer.
Table 1. Therapies with Indications for Skin Cancer
| Therapeutic(s) | BLA/NDA | Indication | Submission Class | Action Date | Skin Neoplasia | Primary Endpoint | Major Efficacy Outcome Measure | Subjects Enrolled | Lower Bound 95% CI (%) | Assessment Tool | Accelerated Approval | Mechanism |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MECHLORETHAMINE HYDROCHLORIDE | NDA006695 | Mycosis fungoides | Type 1 - New Molecular Entity | 1949-03-15 | CTCL | NA | NA | NA | NA | NA | No | Alkylating Agent |
| CORTISONE ACETATE | NDA007110 | Mycosis fungoides | Type 1 - New Molecular Entity | 1950-06-13 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| CORTISONE ACETATE | NDA007750 | Mycosis fungoides | Type 3 - New Dosage Form | 1950-12-04 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| HYDROCORTISONE | NDA008697 | Mycosis fungoides | Type 5 - New Formulation or New Manufacturer | 1952-12-15 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| METHOTREXATE SODIUM | NDA008085 | Mycosis fungoides | Type 1 - New Molecular Entity | 1953-12-07 | CTCL | Response Rate | NA | NA | NA | NA | No | Antimetabolite |
| PREDNISONE | NDA009766 | Mycosis fungoides | Type 1 - New Molecular Entity | 1955-02-21 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| HYDROCORTISONE SODIUM SUCCINATE | NDA009866 | Mycosis fungoides | Type 2 - New Active Ingredient | 1955-04-27 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| PREDNISOLONE | NDA009987 | Mycosis fungoides | Type 1 - New Molecular Entity | 1955-06-21 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| METHYLPREDNISOLONE | NDA011153 | Mycosis fungoides | Type 1 - New Molecular Entity | 1957-10-24 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| TRIAMCINOLONE | NDA011161 | Mycosis fungoides | Type 1 - New Molecular Entity | 1957-12-03 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| DEXAMETHASONE | NDA011664 | Mycosis fungoides | Type 1 - New Molecular Entity | 1958-10-30 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| TRIAMCINOLONE DIACETATE | NDA011685 | Mycosis fungoides | Type 2 - New Active Ingredient | 1959-03-12 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| METHYLPREDNISOLONE SODIUM SUCCINATE | NDA011856 | Mycosis fungoides | Type 2 - New Active Ingredient | 1959-05-18 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| METHYLPREDNISOLONE ACETATE | NDA011757 | Mycosis fungoides | Type 2 - New Active Ingredient | 1959-05-27 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| METHOTREXATE SODIUM | NDA011719 | Mycosis fungoides | Type 2 - New Active Ingredient | 1959-08-10 | CTCL | Response Rate | NA | NA | NA | NA | No | Antimetabolite |
| DEXAMETHASONE SODIUM PHOSPHATE | NDA012071 | Mycosis fungoides | Type 3 - New Dosage Form | 1959-10-06 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| CYCLOPHOSPHAMIDE | NDA012142 | Mycosis fungoides | Type 2 - New Active Ingredient | 1959-11-16 | CTCL | NA | NA | NA | NA | NA | No | Alkylating Agent |
| CYCLOPHOSPHAMIDE | NDA012141 | Mycosis fungoides | Type 1 - New Molecular Entity | 1959-11-16 | CTCL | NA | NA | NA | NA | NA | No | Alkylating Agent |
| METHYLPREDNISOLONE ACETATE | NDA012421 | Mycosis fungoides | Type 3 - New Dosage Form | 1960-06-21 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| DEXAMETHASONE | NDA012376 | Mycosis fungoides | Type 3 - New Dosage Form | 1960-07-07 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| BETAMETHASONE | NDA012657 | Mycosis fungoides | Type 1 - New Molecular Entity | 1961-04-17 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| TRIAMCINOLONE DIACETATE | NDA012802 | Mycosis fungoides | Type 5 - New Formulation or New Manufacturer | 1961-09-05 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| TRIAMCINOLONE ACETONIDE | NDA014901 | Mycosis fungoides | Type 5 - New Formulation or New Manufacturer | 1965-02-01 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| BETAMETHASONE ACETATE | NDA014602 | Mycosis fungoides | Type 1 - New Molecular Entity | 1965-03-03 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| VINBLASTINE SULFATE | NDA012665 | Mycosis fungoides | Type 1 - New Molecular Entity | 1965-11-25 | CTCL | NA | NA | NA | NA | NA | No | Microtubule Inhibitor |
| VINBLASTINE SULFATE | NDA012665 | Kaposi's sarcoma | Type 1 - New Molecular Entity | 1965-11-25 | KS | NA | NA | NA | NA | NA | No | Microtubule Inhibitor |
| HYDROXYUREA | NDA016295 | Melanoma | Type 1 - New Molecular Entity | 1967-12-07 | Melanoma | Overall Response Rate | NA | NA | NA | NA | No | Antimetabolite |
| FLUOROURACIL | NDA016831 | Multiple actinic keratoses | Type 3 - New Dosage Form | 1970-07-29 | AK | NA | NA | NA | NA | NA | NA | Antimetabolite |
| FLUOROURACIL | NDA016988 | Actinic keratoses | Type 5 - New Formulation or New Manufacturer | 1971-08-06 | AK | NA | NA | NA | NA | NA | No | Antimetabolite |
| DACARBAZINE | NDA017575 | Metastatic malignant melanoma | Type 1 - New Molecular Entity | 1975-05-27 | Melanoma | Overall Response Rate | NA | NA | NA | NA | No | Alkylating Agent |
| FLUOROURACIL | NDA016831 | Superficial basal cell carcinoma | Efficacy | 1975-06-30 | BCC | Complete Clearance Rate | CCR: 93% | 54 | NA | NA | No | Antimetabolite |
| PREDNISOLONE SODIUM PHOSPHATE | NDA019157 | Mycosis fungoides | Type 3 - New Dosage Form | 1986-05-28 | CTCL | Bioequivalence | NA | 12 | NA | PK | No | Glucocorticoid |
| METHOXSALEN | NDA009048 | Refractory cutaneous t-cell lymphoma | Efficacy | 1988-03-23 | CTCL | Response Rate | NA | NA | NA | NA | No | Phototoxic Agent |
| INTERFERON ALFA-2B | BLA103132 | AIDS-related kaposi's sarcoma | Efficacy | 1988-11-21 | KS | Response Rate | RR: 29% (30 million IU study) RR: 30% (35 million IU study) | 144 | NA | NA | No | Cytokine |
| INTERFERON ALFA-2A | BLA103145 | AIDS-related kaposi's sarcoma | Efficacy | 1988-11-21 | KS | Response Rate | RR: 25.5% (35 MIU) | 350 | NA | NA | No | Cytokine |
| DOXORUBICIN HYDROCHLORIDE | NDA050718 | Accelerated approval for refractory AIDS-related kaposi's sarcoma | Type 3 - New Dosage Form | 1995-11-17 | KS | Response Rate | RR: 27% (Investigator Assessment) RR: 48% (Indicator Lesion Assessment) | 77 | NA | ACTG | Yes | Topoisomerase Inhibitor |
| INTERFERON ALFA-2B | BLA103132 | Adjuvant melanoma | Efficacy | 1995-12-05 | Melanoma | Relapse-Free Survival | RFS: 1.72 vs. 0.98 years OS: 3.82 vs. 2.78 years | 280 (T=143 C=137) | NA | Clinical | No | Cytokine |
| DAUNORUBICIN CITRATE | NDA050704 | Hiv-associated kaposi's sarcoma - first line | Type 3 - New Dosage Form | 1996-04-08 | KS | Response Rate | RR: 23% | 227 (T=116 C=111) | NA | ACTG | No | Topoisomerase Inhibitor |
| PACLITAXEL | NULL | AIDS-related kaposi's sarcoma - second line | Efficacy | 1997-08-04 | KS | Response Rate | RR: 59% | 59 | NA | ACTG | No | Microtubule Stabilizer |
| ALDESLEUKIN | BLA103293 | Metastatic melanoma | Efficacy | 1998-01-09 | Melanoma | Objective Response Rate | RR: 15.9% | 270 | NA | 2D Measurement | No | Cytokine |
| PREDNISOLONE SODIUM PHOSPHATE | NDA019157 | Expansion to include pediatric populations | Efficacy | 1998-12-17 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| ALITRETINOIN | NDA020886 | AIDS-related kaposi's sarcoma - cutaneous lesions only | Type 1 - New Molecular Entity | 1999-02-02 | KS | Response Rate | RR: 35% (Study 1) RR: 36% (Study 2) | 350 (T=170 C=180) | NA | ACTG | No | Retinoid |
| DENILEUKIN DIFTITOX | BLA103767 | Accelerated approval for persistent or recurrent CD25+ cutaneous t-cell lymphoma | Type 1 - New Molecular Entity | 1999-02-05 | CTCL | Response Rate | RR: 36% (18 mcg) RR: 23% (9 mcg) | 71 (18mcg=36 9mcg=35) | NA | SWAT | Yes | Cytokine-Cytotoxin |
| METHOXSALEN | NDA020969 | Refractory cutaneous t-cell lymphoma | Type 3 - New Dosage Form | 1999-02-25 | CTCL | Response Rate | RR: 33.3% | 51 | 25 | Composite | No | Phototoxic Agent |
| AMINOLEVULINIC ACID HYDROCHLORIDE | NDA020965 | Non-hyperkeratotic actinic keratoses of the face or scalp | Type 1 - New Molecular Entity | 1999-12-03 | AK | Complete Response Rate | CCR: 69% (Trial 1) CCR: 63% (Trial 2) | 241 (T=180 C=61) | NA | Clinical | No | Phototoxic Agent |
| BEXAROTENE | NDA021055 | Refractory cutaneous t-cell lymphoma | Type 1 - New Molecular Entity | 1999-12-29 | CTCL | Response Rate | RR: 32.2% | 152 | 5 | Composite | No | Retinoid |
| BEXAROTENE | NDA021056 | Refractory stage ia and ib cutaneous t-cell lymphoma | Type 3 - New Dosage Form | 2000-06-28 | CTCL | Response Rate | RR: 26% | 50 | 5 | Composite | No | Retinoid |
| DICLOFENAC SODIUM | NULL | Actinic keratoses | Type 3 - New Dosage Form | 2000-10-16 | AK | Complete Clearance Rate | CCR: 47% (Study 1 90d Tx) CCR: 34% (Study 2 90d Tx) CCR: 31% (Study 3 60d Tx) CCR: 14% (Study 3 30d Tx) | 427 (T=213 C=214) | NA | Clinical | No | NSAID |
| FLUOROURACIL | NDA020985 | Multiple actinic keratoses of the face and anterior scalp | Type 3 - New Dosage Form | 2000-10-27 | AK | Complete Clearance Rate | CCR: 58% (Study 1) CCR: 38% (Study 2) | 384 (T=257 C=127) | NA | Clinical | No | Antimetabolite |
| IMIQUIMOD | NDA020723 | Nonhypertrophic actinic keratoses on the face or scalp | Efficacy | 2004-03-02 | AK | Complete Clearance Rate | CRR: 46% (Study A) CCR: 44% (Study B) | 436 (T=215 C=221) | NA | Clinical | No | TLR Agonist |
| IMIQUIMOD | NDA020723 | Superficial basal cell carcinoma | Efficacy | 2004-07-14 | BCC | Complete Clearance Rate | CCR: 70% (Study C) CCR: 80% (Study D) | 364 (T=185 C=179) | NA | Composite | No | TLR Agonist |
| METHYL AMINOLEVULINATE HYDROCHLORIDE | NDA021415 | Non-keratotic actinic keratoses of the face and scalp | Type 3 - New Dosage Form | 2004-07-27 | AK | Complete Response Rate | CRR: 81% (Australian Study) CCR: 79% (U.S. Study) | 191 (T=130 C=61) | NA | Clinical | No | Phototoxic Agent |
| PREDNISOLONE SODIUM PHOSPHATE | NDA021959 | Mycosis fungoides | Type 3 - New Dosage Form | 2006-06-01 | CTCL | Bioequivalence | NA | NA | NA | PK | No | Glucocorticoid |
| VORINOSTAT | NDA021991 | Refractory cutaneous t-cell lymphoma | Type 1 - New Molecular Entity | 2006-10-06 | CTCL | Response Rate | RR: 29.7% (Study 1) RR: 24.2% (Study 2) | 107 | 5 | mSWAT | No | HDAC Inhibitor |
| IMATINIB MESYLATE | NDA021588 | Unresectable, recurrent and/or metastatic dermatofibrosarcoma protuberans | Efficacy | 2006-10-19 | DFSP | Overall Response Rate | RR: 83.3% | 18 | NA | NA | No | Kinase Inhibitor |
| PREDNISOLONE ACETATE | NDA022067 | Mycosis fungoides | Type 3 - New Dosage Form | 2008-01-17 | CTCL | Bioequivalence | NA | 72 | NA | NA | No | Glucocorticoid |
| DOXORUBICIN HYDROCHLORIDE | NDA050718 | Regular aprroval for refractory AIDS-related kaposi's sarcoma | Efficacy | 2008-06-10 | KS | Overall Response Rate | RR: 41% (Study 1) RR: 36% (Study 2) | 28 | NA | NA | No | Topoisomerase Inhibitor |
| TRIAMCINOLONE ACETONIDE | NDA022220 | Mycosis fungoides | Type 3 - New Dosage Form | 2008-06-16 | CTCL | NA | NA | NA | NA | NA | No | Glucocorticoid |
| METHYL AMINOLEVULINATE HYDROCHLORIDE | NDA021415 | Use of metvixia with a new lamp (aktilite) in AKs | Efficacy | 2008-06-26 | AK | Complete Response Rate | CCR: 59.2% (Study 1) CCR: 68.4% (Study 2) | 211 (T=106 C=105) | NA | Clinical | No | Phototoxic Agent |
| DENILEUKIN DIFTITOX | BLA103767 | Regular approval for persistent or recurrent CD25+ cutaneous t-cell lymphoma | Efficacy | 2008-10-15 | CTCL | Response Rate | RR: 46% (18 mcg) RR: 37% (9 mcg) | 144 (18mcg=55 9mcg=45 C=44) | NA | WSSI | No | Cytokine-Cytotoxin |
| ROMIDEPSIN | NDA022393 | Refractory cutaneous t-cell lymphoma | Type 1 - New Molecular Entity | 2009-11-05 | CTCL | Objective Response Rate | RR: 34% (Pivotal Study) RR: 35% (Supportive Study) | 167 | 15 | Composite | No | HDAC Inhibitor |
| AMINOLEVULINIC ACID HYDROCHLORIDE | NDA020965 | Allow user to mix contents for 30 seconds prior to use & to use 'kerastick krusher' | Efficacy | 2010-03-12 | AK | NA | NA | NA | NA | NA | No | Phototoxic Agent |
| IMIQUIMOD | NDA022483 | Use of 3.75% form for actinic keratoses of the full face or balding scalp | Type 5 - New Formulation or New Manufacturer | 2010-03-25 | AK | Complete Clearance Rate | CCR: 25.9% (Study 1) CCR: 45.6% (Study 2) | 319 (T=160 C=159) | NA | Clinical | No | TLR Agonist |
| IPILIMUMAB | BLA125377 | Unresectable or metastatic melanoma | Type 1 - New Molecular Entity | 2011-03-25 | Melanoma | Overall Survival | HR: 0.66 | 676 (I=137 IGP=403 GP=136) | NA | Clinical | No | CTLA-4 Targeted Antibody |
| PEGINTERFERON ALFA-2B | BLA103949 | Adjuvant melanoma | Efficacy | 2011-03-29 | Melanoma | Relapse-Free Survival | HR: 0.82 | 1256 (T=627 C=629) | NA | Clinical | No | Cytokine |
| IMIQUIMOD | NDA022483 | Use of 2.5% cream for AKs on face and scalp | Efficacy | 2011-07-15 | AK | Complete Clearance Rate | CCR: 23% (Study 1) CCR: 38% (Study 2) | 319 (T=160 C=159) | NA | Clinical | No | TLR Agonist |
| VEMURAFENIB | NDA202429 | Unresectable or metastatic melanoma | Type 1 - New Molecular Entity | 2011-08-17 | Melanoma | Overall Survival & Progression-Free Survival | HR: 0.44 (OS) HR: 0.26 (PFS) | 675 (T=337 C=338) | NA | RECIST | No | Kinase Inhibitor |
| INGENOL MEBUTATE | NULL | Actinic keratoses | Type 1 - New Molecular Entity | 2012-01-23 | AK | Complete Clearance Rate | CCR: 37% (Study 1 Head and Neck) CCR: 47% (Study 2 Head & Neck) CCR: 28% (Study 3 Trunk & Extremities) CCR: 42% (Study 4 Trunk & Extremities) | 1005 (H/N T=277 C=270 T/E T=226 C=232) | NA | Clinical | No | Cytotoxic Agent |
| VISMODEGIB | NDA203388 | Locally advanced or metastatic basal cell carcinoma | Type 1 - New Molecular Entity | 2012-01-30 | BCC | Objective Response Rate | RR: 30.3% (mBCC) RR: 42.9% (laBCC) | 96 (mBCC=33 laBCC=63) | 10 20 | mRECIST | No | Smoothened Inhibitor |
| TRAMETINIB DIMETHYL SULFOXIDE | NDA204114 | Unresectable or metastatic BRAF-mutated melanoma | Type 1 - New Molecular Entity | 2013-05-29 | Melanoma | Progression-Free Survival | HR: 0.47 | 322 (T=214 C=108) | NA | RECIST | No | Kinase Inhibitor |
| DABRAFENIB MESYLATE | NDA202806 | Single agent, first-line in advanced BRAF-mutated melanoma | Type 1 - New Molecular Entity | 2013-05-29 | Melanoma | Progression-Free Survival | HR: 0.33 | 250 (T=187 C=63) | NA | RECIST | No | Kinase Inhibitor |
| MECHLORETHAMINE HYDROCHLORIDE | NDA202317 | Refractory stage ia and ib cutaneous t-cell lymphoma | Type 5 - New Formulation or New Manufacturer | 2013-08-23 | CTCL | Response Rate | RR: 60% | 242 (T=119 C=123) | NA | Composite | No | Alkylating Agent |
| TRAMETINIB DIMETHYL SULFOXIDE | NDA204114 | Unresectable or metastatic BRAF-mutated melanoma | Efficacy | 2014-01-08 | Melanoma | Objective Response Rate & Progression-Free Survival | ORR: 76% | 162 (DT2=55 DT1=54 D=53) | NA | RECIST | Yes | Kinase Inhibitor |
| DABRAFENIB MESYLATE | NDA202806 | Accelerated approval for dab/tram in advanced BRAF mutant melanoma | Efficacy | 2014-01-09 | Melanoma | Objective Response Rate | RR: 76% | 162 (DT2=55 DT1=54 D=53) | NA | RECIST | Yes | Kinase Inhibitor |
| PEMBROLIZUMAB | BLA125514 | Unresectable or metastatic melanoma - second line | Type 1 - New Molecular Entity | 2014-09-04 | Melanoma | Overall Response Rate | RR: 23.6% | 173 | 10 | mRECIST | Yes | PD-1 Targeted Antibody |
| NIVOLUMAB | BLA125554 | Accelerated approval for unresectable or metastatic melanoma in the second-line setting | Type 1 - New Molecular Entity | 2014-12-22 | Melanoma | Overall Response Rate | RR: 32% | 120 | 15 | RECIST | Yes | PD-1 Targeted Antibody |
| SONIDEGIB PHOSPHATE | NDA205266 | Locally advanced basal cell carcinoma | Type 1 - New Molecular Entity | 2015-07-24 | BCC | Objective Response Rate | RR: 57.5% | 194 | 20 | mRECIST | No | Smoothened Inhibitor |
| NIVOLUMAB | BLA125554 | Accelerated approval of OPDIVO with yervoy in BRAF wild type unresectable or metastatic melanoma | Efficacy | 2015-09-30 | Melanoma | Objective Response Rate | RR: 60% | 109 (T=72 C=37) | NA | RECIST | Yes | PD-1 Targeted Antibody |
| TALIMOGENE LAHERPAREPVEC | BLA125518 | Unresectable cutaneous, subcutaneous, and nodal recurrent melanoma | Type 1 - New Molecular Entity | 2015-10-27 | Melanoma | Durable Response Rate | RR: 16.3% | 436 (T=295 C=141) | NA | WHO | No | Oncolytic Virus |
| IPILIMUMAB | BLA125377 | Adjuvant melanoma | Efficacy | 2015-10-28 | Melanoma | Relapse-Free Survival | HR: 0.75 | 951 (T=475 C=476) | NA | Clinical | No | CTLA-4 Targeted Antibody |
| COBIMETINIB FUMARATE | NDA206192 | Unresectable or metastatic melanoma with a BRAF-mutated melanoma in combination with vemurafenib | Type 1 - New Molecular Entity | 2015-11-10 | Melanoma | Progression-Free Survival | HR: 0.56 | 495 (T=247 C=248) | NA | RECIST | No | Kinase Inhibitor |
| INGENOL MEBUTATE | NULL | Describing response to a repeat course | Efficacy | 2015-11-19 | AK | Complete Clearance Rate | NA | NA | NA | Clinical | No | Cytotoxic Agent |
| TRAMETINIB DIMETHYL SULFOXIDE | NDA204114 | Unresectable or metastatic melanoma with a BRAF-mutated melanoma in combination with daBRAFenib | Efficacy | 2015-11-20 | Melanoma | Progression-Free Survival | HR: 0.75 | 423 (DT=211 DP=212) | NA | RECIST | No | Kinase Inhibitor |
| DABRAFENIB MESYLATE | NDA202806 | Regular approval for dab/tram in advanced BRAF mutant melanoma | Efficacy | 2015-11-20 | Melanoma | Overall Survival | HR: 0.71 (Trial 2) HR: 0.69 (Trial 3) | 1127 (Trial 2 DT=211 DP=212 Trial 3 DT=352 Vem=352) | NA | RECIST | No | Kinase Inhibitor |
| NIVOLUMAB | BLA125554 | Regular approval for unresectable or metastatic BRAF-wild type melanoma | Efficacy | 2015-11-23 | Melanoma | Overall Survival | HR: 0.42 | 418 (T=210 C=208) | NA | RECIST | No | PD-1 Targeted Antibody |
| PEMBROLIZUMAB | BLA125514 | Unresectable or metastatic melanoma | Efficacy | 2015-12-18 | Melanoma | Progression-Free Survival & Overall Survival | HR: 0.57 (2 mg/kg) HR: 0.50 (10 mg/kg) | 540 (P2=180 P10=181 C=179) | NA | mRECIST | No | PD-1 Targeted Antibody |
| PEMBROLIZUMAB | BLA125514 | Removing language limiting the indication to disease progression following ipilimumab | Efficacy | 2015-12-18 | Melanoma | Progression-Free Survival & Overall Survival | HR: 0.69 (OS) HR: 0.58 (PFS) | 834 (T=556 C=278) | NA | mRECIST | No | PD-1 Targeted Antibody |
| NIVOLUMAB | BLA125554 | Accelerated approved for BRAF mutant unresectable or metastatic melanoma in the first line setting | Efficacy | 2016-01-23 | Melanoma | Progression-Free Survival & Overall Survival | HR: 0.57 (BRAF wt & mutant) HR: 0.77 (BRAF mutant only) | 198 (T=98 C=100) | NA | RECIST | Yes | PD-1 Targeted Antibody |
| NIVOLUMAB | BLA125554 | Accelerated approval of ipi/nivo expansion for BRAF mutant as well as BRAF wt | Efficacy | 2016-01-23 | Melanoma | Progression-Free Survival & Overall Survival | HR: 0.42 (BRAF wt & mut) HR: 0.47 (BRAF mut only) | 198 (T=98 C=100) | NA | RECIST | Yes | PD-1 Targeted Antibody |
| AMINOLEVULINIC ACID HYDROCHLORIDE | NDA208081 | Actinic keratoses of mild-to-moderate severity on the face and scalp | Type 3 - New Dosage Form | 2016-05-10 | AK | Complete Response Rate | CCR: 85% (Trial 1) CCR: 84% (Trial 2) CCR: 91% (Trial 3) | 299 (T=212 C=87) | NA | Clinical | No | Phototoxic Agent |
| NIVOLUMAB | BLA125554 | Flat dosing for melanoma, NSCLC and RCC | Efficacy | 2016-09-13 | Melanoma | NA | NA | NA | NA | NA | No | PD-1 Targeted Antibody |
| NIVOLUMAB | BLA125554 | Flat dosing for melanoma, NSCLC and RCC | Efficacy | 2016-09-13 | Melanoma | NA | NA | NA | NA | NA | No | PD-1 Targeted Antibody |
| AVELUMAB | BLA761049 | Metastatic merkel cell carcinoma | Type 1 - New Molecular Entity | 2017-03-23 | MCC | Overall Response Rate | RR: 33% | 88 | 20 | RECIST | Yes | PD-L1 Targeted Antibody |
| PEMBROLIZUMAB | BLA125514 | Flat dosing of 200 mg q3 weeks in melanoma | Efficacy | 2017-05-17 | Melanoma | NA | NA | NA | NA | NA | No | PD-1 Targeted Antibody |
| PEMBROLIZUMAB | BLA125514 | Unresectable or metastatic, microsatellite instability-high or mismatch repair deficient solid tumors | Efficacy | 2017-05-23 | Agnostic | Objective Response Rate & Duration of Response | RR: 39.6% DoR: Not Reached | 149 | NA | mRECIST | Yes | PD-1 Targeted Antibody |
| IPILIMUMAB | BLA125377 | Unresectable or metastatic melanoma - patients 12 years and older | Efficacy | 2017-07-21 | Melanoma | Pharmacokinetic Analysis | NA | 45 | NA | PK | No | CTLA-4 Targeted Antibody |
| BRENTUXIMAB VEDOTIN | BLA125388 | CD30+ mycosis fundgoides and pcalcl in the second line | Efficacy | 2017-11-09 | CTCL | Durable Response Rate | RR: 56.3% | 128 (T=66 C=62) | NA | GRS | No | CD30 Targeted Antibody |
| NIVOLUMAB | BLA125554 | Regular approval for adjuvant melanoma | Efficacy | 2017-12-20 | Melanoma | Relapse-Free Survival | HR: 0.65 | 906 (T=453 C=453) | NA | Clinical | No | PD-1 Targeted Antibody |
| NIVOLUMAB | BLA125554 | Updates infusion from 60 to 30 minutes | Efficacy | 2018-01-09 | Melanoma | NA | NA | NA | NA | NA | No | PD-1 Targeted Antibody |
| COBIMETINIB FUMARATE | NDA206192 | Updates label to include OS data | Efficacy | 2018-01-26 | Melanoma | NA | NA | NA | NA | NA | No | Kinase Inhibitor |
| NIVOLUMAB | BLA125554 | Every 4 week dosing for advanced melanoma | Efficacy | 2018-03-05 | Melanoma | Pharmacokinetic Analysis | NA | 4166 | NA | PK | No | PD-1 Targeted Antibody |
| NIVOLUMAB | BLA125554 | Every 4 week dosing in adjuvant melanoma | Efficacy | 2018-03-05 | Melanoma | NA | NA | NA | NA | NA | No | PD-1 Targeted Antibody |
| NIVOLUMAB | BLA125554 | Updates infusion from 60 to 30 minutes in adjuvant melanoma | Efficacy | 2018-03-05 | Melanoma | NA | NA | NA | NA | NA | No | PD-1 Targeted Antibody |
| AMINOLEVULINIC ACID HYDROCHLORIDE | NDA020965 | Minimally to moderately thick actinic keratosis of the upper extremities | Efficacy | 2018-03-06 | AK | Complete Response Rate | CCR: 31% | 269 (T=135 C=134) | NA | NA | No | Phototoxic Agent |
| TRAMETINIB DIMETHYL SULFOXIDE | NDA204114 | Adjuvant BRAF mutant melanoma in combination with daBRAFenib | Efficacy | 2018-04-30 | Melanoma | Relapse-Free Survival | HR: 0.47 | 870 (T=438 C=432) | NA | Clinical | No | Kinase Inhibitor |
| DABRAFENIB MESYLATE | NDA202806 | Adjuvant BRAF mutant melanoma in combination with trametinib | Efficacy | 2018-04-30 | Melanoma | Relapse-Free Survival | HR: 0.47 | 870 (T=438 C=432) | NA | Clinical | No | Kinase Inhibitor |
| ENCORAFENIB | NDA210496 | Regular approval for use in combination with binimetinib for advanced BRAF mutant melanoma | Type 1 - New Molecular Entity and Type 4 - New Combination | 2018-06-27 | Melanoma | Progression-Free Survival | HR: 0.54 | 577 (Bin/Enc=192 Enc=192 Vem=191) | NA | RECIST | No | Kinase Inhibitor |
| BINIMETINIB | NDA210498 | Unresectable or metastatic BRAF-mutated melanoma in combination with encorafenib | Type 1 - New Molecular Entity and Type 4 - New Combination | 2018-06-27 | Melanoma | Progression-Free Survival | HR: 0.54 | 577 (Bin/Enc=192 Enc=192 Vem=191) | NA | RECIST | No | Kinase Inhibitor |
| MOGAMULIZUMAB-KPKC | BLA761051 | Relapsed/refractory mycosis fungoides or sezary syndrome - second line | Type 1 - New Molecular Entity | 2018-08-08 | CTCL | Progression-Free Survival | HR: 0.53 | 372 (T=186 C=186) | NA | GRS | No | CCR4 Targeted Antibody & ADCC Inducer |
| CEMIPLIMAB-RWLC | BLA761097 | Locally advanced or metastatic squamous cell carcinoma | Type 1 - New Molecular Entity | 2018-09-28 | SCC | Overall Response Rate | RR: 46.7% (mCSCC) RR: 48.5% (laCSCC) | 108 (mSCC=75 laSCC=33) | 15 25 | Composite | No | PD-1 Targeted Antibody |
| PEMBROLIZUMAB | BLA125514 | Locally advanced or metastatic merkel cell carcinoma | Efficacy | 2018-12-19 | MCC | Overall Response Rate & Duration of Response | RR: 56% DoR: 5.9-34.5+ (months) | 50 | NA | RECIST | Yes | PD-1 Targeted Antibody |
| VISMODEGIB | NDA203388 | To allow for treatment interruptions of up to 8 weeks for intolerable aes | Efficacy | 2019-01-18 | BCC | NA | NA | NA | NA | NA | No | Smoothened Inhibitor |
| PEMBROLIZUMAB | BLA125514 | Adjuvant melanoma | Efficacy | 2019-02-15 | Melanoma | Relapse-Free Survival | HR: 0.57 | 1019 (T=509 C=502) | NA | Clinical | No | PD-1 Targeted Antibody |
| NIVOLUMAB | BLA125554 | Conversion to regular approval for the treatment of patients with BRAF v600 mutation-positive unresectable or metastatic melanoma | Efficacy | 2019-03-07 | Melanoma | Overall Survival | NA | 405 (T=272 C=133) | NA | NA | No | PD-1 Targeted Antibody |
| NIVOLUMAB | BLA125554 | Conversion to regular approval of OPDIVO, in combination with ipilimumab, for the treatment of patients with unresectable or metastatic melanoma | Efficacy | 2019-03-07 | Melanoma | Overall Survival | NA | 945 (IN=314 N=316 I=315) | NA | Clinical | No | PD-1 Targeted Antibody |
| TRAMETINIB DIMETHYL SULFOXIDE | NDA204114 | Updating results in subjects with brain metastases | Efficacy | 2019-10-06 | Melanoma | Overall Response Rate | RR: 50% | 121 | NA | mRECIST | No | Kinase Inhibitor |
| DABRAFENIB MESYLATE | NDA202806 | Updates label in regards to brain metastases for melanoma | Efficacy | 2019-10-06 | Melanoma | Overall Response Rate | RR: 50% | 121 | NA | mRECIST | No | Kinase Inhibitor |
| PEMBROLIZUMAB | BLA125514 | Every 6 weeks dosing in melanoma | Efficacy | 2020-04-28 | Melanoma | Pharmacokinetic Analysis | NA | 44 | NA | PK | Yes | PD-1 Targeted Antibody |
| PEMBROLIZUMAB | BLA125514 | Every 6 weeks dosing in merkel cell carcinoma | Efficacy | 2020-04-28 | MCC | NA | NA | NA | NA | NA | Yes | PD-1 Targeted Antibody |
| PEMBROLIZUMAB | BLA125514 | Every 6 weeks dosing in msi-h tumors | Efficacy | 2020-04-28 | Agnostic | NA | NA | NA | NA | NA | Yes | PD-1 Targeted Antibody |
| POMALIDOMIDE | NDA204026 | Treatment of adult patients with AIDS-related kaposi sarcoma | Efficacy | 2020-05-14 | KS | Objective Response Rate | RR: 67% | 28 (HIV+=18 HIV-=10) | NA | ACTG | Yes | Cereblon Inhibitor |
| POMALIDOMIDE | NDA204026 | Treatment of kaposi’s sarcoma in patients who are hiv-negative | Efficacy | 2020-05-14 | KS | Objective Response Rate | RR: 80% | 28 (HIV+=18 HIV-=10) | NA | ACTG | Yes | Cereblon Inhibitor |
| PEMBROLIZUMAB | BLA125514 | Tumor mutation burden-high solid tumors | Efficacy | 2020-06-16 | Agnostic | Objective Response Rate & Duration of Response | RR: 29% | 102 | NA | mRECIST | Yes | PD-1 Targeted Antibody |
| PEMBROLIZUMAB | BLA125514 | Every 6 weeks dosing in TMB-hi tumors | Efficacy | 2020-06-16 | Agnostic | NA | NA | NA | NA | NA | Yes | PD-1 Targeted Antibody |
| PEMBROLIZUMAB | BLA125514 | R/mCSCC | Efficacy | 2020-06-24 | SCC | Objective Response Rate | RR: 34.3% | 105 | 15 | mRECIST | No | PD-1 Targeted Antibody |
| PEMBROLIZUMAB | BLA125514 | Every 6 weeks dosing for CSCC | Efficacy | 2020-06-24 | SCC | NA | NA | NA | NA | NA | Yes | PD-1 Targeted Antibody |
| ATEZOLIZUMAB | BLA761034 | Unresectable or metastatic BRAF-mutated melanoma in combination with cobimetinib and vemurafenib | Efficacy | 2020-07-30 | Melanoma | Progression-Free Survival | HR: 0.78 | 514 (T=256 C=258) | NA | RECIST | No | PD-L1 Targeted Antibody |
| TIRBANIBULIN | NDA213189 | Actinic keratosis of the face or scalp | Type 1 - New Molecular Entity | 2020-12-14 | AK | Complete Clearance Rate | CCR: 44% (Study 1) CCR: 54% (Study 2) | 702 (T=353 C=349) | NA | Clinical | No | Microtubule Inhibitor |
| CEMIPLIMAB-RWLC | BLA761097 | Locally advanced basal cell carcinoma | Efficacy | 2021-02-09 | BCC | Objective Response Rate | RR: 29% | 112 (laBCC=84) | 20 | Composite | No | PD-1 Targeted Antibody |
| CEMIPLIMAB-RWLC | BLA761097 | Metastatic basal cell carcinoma | Efficacy | 2021-02-09 | BCC | Objective Response Rate | RR: 21% | 112 (mBCC=28) | NA | Composite | Yes | PD-1 Targeted Antibody |
The above table lists the therapeutic agents (or agents) that were the subject of the submitted application. Key data, such as the NDA/BLA number, submission class, action date, disease for which the labeled indication is directed (“Skin Neoplasia”), the primary endpoint of the pivotal trial, the major efficacy outcome measure, the number of subjects enrolled in the pivotal trial, the lower bound 95% confidence interval proposed by the sponsor as the minimally effective response rate demonstrative of efficacy, mechanism, trial design, tools used for primary endpoint assessment, approval pathway, and action number in regard to skin cancer actions are listed. “Non-placebo comparator” connotes either an active comparator (e.g., an alternate standard of care therapy) or multiple dose levels of the investigational agent. The number of Subjects Enrolled for the specific submission are provided along with a parenthetical notation of relevant treatment groups in the study. For example, submissions with comparator groups are listed with the convention T=x and C=x to connote the number of subjects in the “treatment” and “control/comparator” groups, respectively. Additional clarifications are provided in the parentheticals in this column. For example, the approvals for the 02-09-2021 supplement for cemiplimab-RWLC and the 05-14-2020 supplement of pomalidomide are reported on individual rows in the table for the distinct labeled indications (e.g. laBCC/mBCC and HIV-negative/HIV-positive KS, respectively) to highlight the differences in indication. However, the number of subjects listed in the column “Subjects Enrolled” represents the total number of subjects evaluated for that submission to reflect the totality of evidence for which the approvals were made, with the number of subjects with that specific disease subgroup appearing in the parenthetical. Abbreviations. AEs: adverse events, AK: actinic keratosis, BCC: basal cell carcinoma, Bin/Enc: binimetinib/encorafenib, C: comparator/control, CCR: complete clearance rate, CI: confidence interval, CTCL: cutaneous T-cell lymphoma, DFSP: dermatofibrosarcoma protuberans, DoR: duration of response, D: dabrafenib, DP: dabrafenib/placebo, DT: dabrafenib/trametinib, DT1: dabrafenib/trametinib 1mg, DT2: dabrafenib/trametinib 2mg, Enc: encorafenib, GP: gp100, HIV: human immunodeficiency virus, H/N: head and neck, HR: hazard ratio, I: ipilimumab, IGP: ipilimumab plus gp100, IN: ipilimumab/nivolumab, KS: Kaposi’s sarcoma, laBCC: locally advanced basal cell carcinoma, laCSCC: locally advanced cutaneous squamous cell carcinoma, mBCC: metastatic basal cell carcinoma, MCC: Merkel cell carcinoma, mcg: microgram, mut: mutant, N: nivolumab, NA: missing, ORR: objective/overall response rate, OS: overall survival, P2: pembrolizumab 2mg/kg, P10: pembrolizumab 10mg/kg, PFS: progression-free survival, RFS: recurrence-free survival, RR: response rate, T: treatment, T/E: trunk and extremities, Tx: treatment, r/mCSCC: recurrent/metastatic cutaneous squamous cell carcinoma, SCC: squamous cell carcinoma, wt: wild type, Vem: vemurafenib.
Table 2. Label Modifications to Therapeutics with an Indication in Skin Cancer
| Substance Name | Brand Name | Application Number | Submission Class | Action Date |
|---|---|---|---|---|
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | TYPE 1 | 1949-03-15 |
| CORTISONE ACETATE | CORTONE | NDA007110 | TYPE 1 | 1950-06-13 |
| CORTISONE ACETATE | CORTONE | NDA007750 | TYPE 3 | 1950-12-04 |
| HYDROCORTISONE | CORTEF | NDA008697 | TYPE 5 | 1952-12-15 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | TYPE 1 | 1953-12-07 |
| METHOXSALEN | 8-MOP | NDA009048 | TYPE 1 | 1954-12-03 |
| PREDNISONE | METICORTEN | NDA009766 | TYPE 1 | 1955-02-21 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | TYPE 2 | 1955-04-27 |
| PREDNISOLONE | DELTA-CORTEF | NDA009987 | TYPE 1 | 1955-06-21 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | TYPE 1 | 1957-10-24 |
| TRIAMCINOLONE | ARISTOCORT | NDA011161 | TYPE 1 | 1957-12-03 |
| DEXAMETHASONE | DECADRON | NDA011664 | TYPE 1 | 1958-10-30 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA011685 | TYPE 2 | 1959-03-12 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | TYPE 2 | 1959-05-18 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | TYPE 2 | 1959-05-27 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | TYPE 2 | 1959-08-10 |
| DEXAMETHASONE SODIUM PHOSPHATE | DECADRON | NDA012071 | TYPE 3 | 1959-10-06 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | TYPE 2 | 1959-11-16 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | TYPE 1 | 1959-11-16 |
| METHYLPREDNISOLONE ACETATE | MEDROL ACETATE | NDA012421 | TYPE 3 | 1960-06-21 |
| DEXAMETHASONE | DECADRON | NDA012376 | TYPE 3 | 1960-07-07 |
| BETAMETHASONE | CELESTONE | NDA012657 | TYPE 1 | 1961-04-17 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | TYPE 5 | 1961-09-05 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | TYPE 5 | 1965-02-01 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | TYPE 1 | 1965-03-03 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | TYPE 1 | 1965-11-25 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | TYPE 1 | 1965-11-25 |
| HYDROXYUREA | DROXIA | NDA016295 | TYPE 1 | 1967-12-07 |
| FLUOROURACIL | EFUDEX | NDA016831 | TYPE 3 | 1970-07-29 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | TYPE 5 | 1971-08-06 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | TYPE 1 | 1974-08-07 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1974-09-19 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1974-11-13 |
| METHOXSALEN | 8-MOP | NDA009048 | S | 1974-11-13 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | MANUF (CMC) | 1974-12-17 |
| METHOXSALEN | 8-MOP | NDA009048 | LABELING | 1975-04-04 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1975-05-05 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1975-05-07 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1975-05-07 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1975-05-12 |
| DACARBAZINE | DTIC-DOME | NDA017575 | TYPE 1 | 1975-05-27 |
| FLUOROURACIL | EFUDEX | NDA016831 | EFFICACY | 1975-06-30 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1975-07-22 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1975-08-25 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1975-10-16 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 1975-11-03 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 1975-11-03 |
| DACARBAZINE | DTIC-DOME | NDA017575 | MANUF (CMC) | 1975-11-10 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1975-11-25 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 1975-12-05 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | MANUF (CMC) | 1975-12-24 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1976-01-13 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | S | 1976-01-26 |
| METHYLPREDNISOLONE ACETATE | MEDROL ACETATE | NDA012421 | LABELING | 1976-02-27 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1976-03-25 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1976-06-09 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1976-06-09 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1976-06-10 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1976-06-29 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 1976-06-29 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1976-06-29 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | LABELING | 1976-07-09 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1976-08-03 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | MANUF (CMC) | 1976-08-06 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1976-09-07 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1976-09-07 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1976-09-07 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | MANUF (CMC) | 1976-09-10 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1976-09-14 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1976-09-14 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1976-09-14 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | REMS | 1976-10-18 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1976-10-18 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1976-11-01 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 1976-11-01 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1976-12-21 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1977-01-13 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | MANUF (CMC) | 1977-03-02 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1977-03-17 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1977-03-17 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1977-03-18 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1977-03-22 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1977-03-22 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1977-03-28 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1977-03-29 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1977-04-20 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1977-04-25 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1977-04-26 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1977-04-26 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 1977-04-29 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | LABELING | 1977-04-29 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1977-05-03 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1977-05-16 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1977-07-14 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1977-07-25 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1977-07-25 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1977-07-25 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1977-08-02 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1977-08-10 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1977-08-18 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | LABELING | 1977-09-01 |
| METHYLPREDNISOLONE ACETATE | MEDROL ACETATE | NDA012421 | MANUF (CMC) | 1977-11-11 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 1977-11-16 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1977-11-16 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1977-12-14 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1977-12-14 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1978-01-09 |
| PREDNISONE | METICORTEN | NDA009766 | LABELING | 1978-01-25 |
| HYDROCORTISONE | CORTEF | NDA008697 | LABELING | 1978-02-08 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | LABELING | 1978-04-07 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 1978-04-07 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | LABELING | 1978-04-11 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | MANUF (CMC) | 1978-04-17 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1978-05-23 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | LABELING | 1978-06-12 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1978-07-03 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 1978-07-10 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | LABELING | 1978-07-12 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1978-07-19 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1978-08-08 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1978-08-08 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | S | 1978-08-16 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1978-08-22 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1978-08-28 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1978-08-29 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1978-09-13 |
| PREDNISOLONE | DELTA-CORTEF | NDA009987 | LABELING | 1978-09-25 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 1978-09-25 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 1978-10-11 |
| DEXAMETHASONE SODIUM PHOSPHATE | DECADRON | NDA012071 | LABELING | 1978-10-18 |
| BETAMETHASONE | CELESTONE | NDA012657 | LABELING | 1978-11-08 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1978-11-21 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1978-11-27 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1978-11-28 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1978-12-07 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1978-12-13 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1978-12-14 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1979-01-02 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | MANUF (CMC) | 1979-01-10 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1979-02-15 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1979-02-16 |
| PREDNISONE | METICORTEN | NDA009766 | S | 1979-02-26 |
| DEXAMETHASONE | DECADRON | NDA012376 | MANUF (CMC) | 1979-03-01 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1979-03-14 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | MANUF (CMC) | 1979-04-05 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | MANUF (CMC) | 1979-04-05 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1979-04-30 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 1979-05-01 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1979-05-24 |
| DACARBAZINE | DTIC-DOME | NDA017575 | MANUF (CMC) | 1979-05-24 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | MANUF (CMC) | 1979-05-30 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 1979-06-18 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1979-06-19 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 1979-07-17 |
| BETAMETHASONE | CELESTONE | NDA012657 | MANUF (CMC) | 1979-07-23 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 1979-07-26 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 1979-07-26 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 1979-07-26 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 1979-07-26 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | LABELING | 1979-07-27 |
| CORTISONE ACETATE | CORTONE | NDA007750 | LABELING | 1979-08-01 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1979-08-03 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1979-08-03 |
| TRIAMCINOLONE | ARISTOCORT | NDA011161 | MANUF (CMC) | 1979-08-06 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | LABELING | 1979-08-08 |
| CORTISONE ACETATE | CORTONE | NDA007110 | LABELING | 1979-08-15 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 1979-08-15 |
| DEXAMETHASONE | DECADRON | NDA012376 | LABELING | 1979-08-15 |
| DEXAMETHASONE SODIUM PHOSPHATE | DECADRON | NDA012071 | LABELING | 1979-08-15 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 1979-08-21 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 1979-09-05 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 1979-09-24 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 1979-09-24 |
| CORTISONE ACETATE | CORTONE | NDA007750 | LABELING | 1979-09-25 |
| BETAMETHASONE | CELESTONE | NDA012657 | MANUF (CMC) | 1979-10-03 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1979-10-15 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1979-10-23 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 1979-10-26 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1979-10-30 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1979-10-30 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1979-10-31 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | LABELING | 1979-11-13 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1979-12-03 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1979-12-18 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1980-01-25 |
| CORTISONE ACETATE | CORTONE | NDA007750 | LABELING | 1980-02-01 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 1980-02-04 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 1980-02-04 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 1980-02-05 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1980-02-19 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | LABELING | 1980-02-19 |
| METHYLPREDNISOLONE ACETATE | MEDROL ACETATE | NDA012421 | LABELING | 1980-02-29 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1980-02-29 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1980-03-31 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | LABELING | 1980-04-14 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | LABELING | 1980-04-14 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 1980-04-23 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1980-04-28 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 1980-05-20 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1980-06-11 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | MANUF (CMC) | 1980-06-20 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | MANUF (CMC) | 1980-07-07 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | MANUF (CMC) | 1980-07-29 |
| PREDNISONE | METICORTEN | NDA009766 | MANUF (CMC) | 1980-08-27 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | MANUF (CMC) | 1980-09-18 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1980-09-27 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 1980-09-27 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1980-09-30 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | MANUF (CMC) | 1980-10-03 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA011685 | MANUF (CMC) | 1980-10-06 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | MANUF (CMC) | 1980-10-08 |
| DACARBAZINE | DTIC-DOME | NDA017575 | MANUF (CMC) | 1980-10-27 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1980-12-03 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | MANUF (CMC) | 1981-01-12 |
| DEXAMETHASONE SODIUM PHOSPHATE | DECADRON | NDA012071 | MANUF (CMC) | 1981-02-05 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | MANUF (CMC) | 1981-02-17 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | LABELING | 1981-02-20 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1981-02-27 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1981-03-21 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 1981-04-01 |
| BETAMETHASONE | CELESTONE | NDA012657 | MANUF (CMC) | 1981-04-11 |
| TRIAMCINOLONE | ARISTOCORT | NDA011161 | MANUF (CMC) | 1981-04-27 |
| DACARBAZINE | DTIC-DOME | NDA017575 | EFFICACY | 1981-04-29 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1981-05-11 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | MANUF (CMC) | 1981-07-02 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1981-07-28 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1981-08-11 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1981-10-13 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1981-10-16 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1981-11-16 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1981-12-07 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1981-12-11 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 1981-12-15 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | LABELING | 1981-12-15 |
| METHYLPREDNISOLONE ACETATE | MEDROL ACETATE | NDA012421 | MANUF (CMC) | 1981-12-28 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 1982-02-10 |
| DEXAMETHASONE | DECADRON | NDA012376 | MANUF (CMC) | 1982-02-10 |
| CORTISONE ACETATE | CORTONE | NDA007750 | MANUF (CMC) | 1982-03-02 |
| CORTISONE ACETATE | CORTONE | NDA007110 | MANUF (CMC) | 1982-03-04 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1982-03-04 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | MANUF (CMC) | 1982-03-04 |
| FLUOROURACIL | EFUDEX | NDA016831 | LABELING | 1982-03-10 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1982-03-15 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1982-03-15 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 1982-03-31 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1982-03-31 |
| METHYLPREDNISOLONE ACETATE | MEDROL ACETATE | NDA012421 | LABELING | 1982-04-02 |
| METHOXSALEN | 8-MOP | NDA009048 | LABELING | 1982-05-07 |
| METHOXSALEN | 8-MOP | NDA009048 | LABELING | 1982-05-07 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1982-05-14 |
| DEXAMETHASONE | DECADRON | NDA012376 | LABELING | 1982-05-21 |
| TRIAMCINOLONE | ARISTOCORT | NDA011161 | MANUF (CMC) | 1982-06-11 |
| BETAMETHASONE | CELESTONE | NDA012657 | MANUF (CMC) | 1982-06-30 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 1982-06-30 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 1982-06-30 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1982-07-28 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1982-07-28 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 1982-07-30 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 1982-07-30 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 1982-08-24 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1982-08-30 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 1982-11-04 |
| BETAMETHASONE | CELESTONE | NDA012657 | MANUF (CMC) | 1982-11-23 |
| CORTISONE ACETATE | CORTONE | NDA007110 | MANUF (CMC) | 1982-12-09 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 1983-02-04 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | MANUF (CMC) | 1983-02-21 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1983-02-24 |
| HYDROCORTISONE | CORTEF | NDA008697 | MANUF (CMC) | 1983-03-09 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | LABELING | 1983-03-09 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | LABELING | 1983-03-09 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | LABELING | 1983-03-09 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1983-03-14 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1983-03-15 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 1983-03-15 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 1983-03-22 |
| DACARBAZINE | DTIC-DOME | NDA017575 | LABELING | 1983-04-05 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1983-04-08 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1983-04-08 |
| METHOXSALEN | 8-MOP | NDA009048 | LABELING | 1983-04-21 |
| BETAMETHASONE | CELESTONE | NDA012657 | MANUF (CMC) | 1983-04-26 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | LABELING | 1983-05-03 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 1983-05-11 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 1983-08-04 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1983-08-10 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1983-08-12 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1983-08-12 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1983-08-25 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1984-01-04 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 1984-04-04 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1984-04-05 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 1984-04-13 |
| METHOXSALEN | 8-MOP | NDA009048 | LABELING | 1984-04-16 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 1984-05-03 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1984-05-04 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1984-05-24 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1984-06-14 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1984-06-14 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1984-06-15 |
| DEXAMETHASONE SODIUM PHOSPHATE | DECADRON | NDA012071 | MANUF (CMC) | 1984-06-25 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1984-07-18 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 1984-07-19 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1984-08-10 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1984-08-13 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1984-08-20 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1984-10-17 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1984-12-10 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1985-01-16 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1985-02-27 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1985-03-04 |
| DEXAMETHASONE SODIUM PHOSPHATE | DECADRON | NDA012071 | LABELING | 1985-03-08 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1985-03-14 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1985-03-28 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1985-05-02 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1985-05-06 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1985-05-20 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1985-06-07 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1985-06-10 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | LABELING | 1985-06-14 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1985-06-18 |
| DACARBAZINE | DTIC-DOME | NDA017575 | MANUF (CMC) | 1985-07-15 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1985-07-16 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | LABELING | 1985-07-24 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1985-08-06 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1985-08-30 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1985-08-30 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1985-08-30 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1985-09-24 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 1985-11-26 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 1985-12-04 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 1985-12-04 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1985-12-05 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1985-12-05 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1985-12-10 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 1986-01-15 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | MANUF (CMC) | 1986-01-15 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1986-01-23 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1986-01-31 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1986-02-11 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1986-02-14 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1986-02-14 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1986-03-21 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | LABELING | 1986-03-29 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | LABELING | 1986-05-12 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | LABELING | 1986-05-12 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | LABELING | 1986-05-12 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1986-05-21 |
| DACARBAZINE | DTIC-DOME | NDA017575 | LABELING | 1986-05-22 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | TYPE 3 | 1986-05-28 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1986-05-30 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1986-05-30 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1986-05-30 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | TYPE 1 | 1986-06-04 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 1986-06-10 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 1986-06-30 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1986-07-11 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1986-07-11 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1986-07-11 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 1986-07-11 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 1986-07-11 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 1986-07-11 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 1986-07-11 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | MANUF (CMC) | 1986-08-14 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1986-09-16 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 1986-09-16 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | LABELING | 1986-09-16 |
| TRIAMCINOLONE | ARISTOCORT | NDA011161 | MANUF (CMC) | 1986-09-25 |
| BETAMETHASONE | CELESTONE | NDA012657 | MANUF (CMC) | 1986-10-20 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 1986-11-03 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1986-11-06 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1986-11-14 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 1986-11-19 |
| TRIAMCINOLONE | ARISTOCORT | NDA011161 | MANUF (CMC) | 1986-11-29 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1986-12-08 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1986-12-08 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1986-12-08 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 1986-12-08 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1986-12-08 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1986-12-08 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | MANUF (CMC) | 1987-01-07 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | LABELING | 1987-01-15 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | LABELING | 1987-01-28 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1987-02-19 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | MANUF (CMC) | 1987-02-24 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1987-03-19 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1987-03-19 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1987-03-19 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | MANUF (CMC) | 1987-03-19 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1987-03-25 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1987-03-25 |
| FLUOROURACIL | EFUDEX | NDA016831 | LABELING | 1987-03-26 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1987-03-27 |
| BETAMETHASONE | CELESTONE | NDA012657 | MANUF (CMC) | 1987-04-02 |
| PREDNISONE | METICORTEN | NDA009766 | MANUF (CMC) | 1987-04-02 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1987-04-28 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | EFFICACY | 1987-04-29 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1987-04-29 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1987-04-29 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | EFFICACY | 1987-04-29 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 1987-04-29 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1987-04-29 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | MANUF (CMC) | 1987-05-05 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 1987-07-01 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 1987-07-02 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1987-07-22 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1987-07-22 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 1987-07-23 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1987-08-18 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 1987-08-25 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | LABELING | 1987-09-18 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | MANUF (CMC) | 1987-10-16 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1987-11-20 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 1987-11-30 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1987-12-30 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1988-01-11 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1988-01-12 |
| METHOXSALEN | 8-MOP | NDA009048 | EFFICACY | 1988-03-23 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1988-03-25 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | EFFICACY | 1988-04-07 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1988-04-07 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 1988-04-18 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1988-04-21 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1988-04-28 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 1988-05-03 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1988-05-10 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 1988-05-13 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1988-08-25 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 1988-10-31 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | EFFICACY | 1988-10-31 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | MANUF (CMC) | 1988-10-31 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | EFFICACY | 1988-11-21 |
| INTERFERON ALFA-2A | ROFERON | BLA103145 | EFFICACY | 1988-11-21 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1988-11-30 |
| DACARBAZINE | DTIC-DOME | NDA017575 | MANUF (CMC) | 1988-12-02 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | MANUF (CMC) | 1989-01-05 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | MANUF (CMC) | 1989-01-05 |
| DEXAMETHASONE | DECADRON | NDA012376 | MANUF (CMC) | 1989-01-12 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | MANUF (CMC) | 1989-02-02 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1989-03-01 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1989-03-01 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | LABELING | 1989-03-22 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | LABELING | 1989-03-22 |
| DACARBAZINE | DTIC-DOME | NDA017575 | MANUF (CMC) | 1989-04-19 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | LABELING | 1989-04-24 |
| CORTISONE ACETATE | CORTONE | NDA007750 | LABELING | 1989-05-16 |
| CORTISONE ACETATE | CORTONE | NDA007110 | LABELING | 1989-05-16 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 1989-05-16 |
| DEXAMETHASONE | DECADRON | NDA012376 | LABELING | 1989-05-16 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 1989-05-18 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 1989-06-06 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 1989-06-06 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1989-07-11 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1989-10-02 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA011685 | MANUF (CMC) | 1989-10-12 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | MANUF (CMC) | 1989-10-12 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | MANUF (CMC) | 1989-10-27 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 1989-11-16 |
| CORTISONE ACETATE | CORTONE | NDA007750 | MANUF (CMC) | 1989-11-30 |
| DEXAMETHASONE SODIUM PHOSPHATE | DECADRON | NDA012071 | MANUF (CMC) | 1989-12-11 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | LABELING | 1989-12-14 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 1990-01-16 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 1990-01-18 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | LABELING | 1990-01-18 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 1990-03-29 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1990-04-24 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 1990-05-01 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 1990-06-18 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 1990-08-10 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 1990-10-05 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 1990-10-22 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1990-10-24 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 1990-10-24 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | MANUF (CMC) | 1990-11-15 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1990-12-07 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1991-02-20 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 1991-04-01 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 1991-07-19 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | LABELING | 1991-07-24 |
| DACARBAZINE | DTIC-DOME | NDA017575 | MANUF (CMC) | 1991-07-29 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | LABELING | 1991-09-04 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1991-11-05 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | MANUF (CMC) | 1992-01-06 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | MANUF (CMC) | 1992-01-22 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1992-01-23 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA011685 | MANUF (CMC) | 1992-02-06 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA011685 | MANUF (CMC) | 1992-02-06 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | MANUF (CMC) | 1992-02-06 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 1992-02-06 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 1992-02-14 |
| ALDESLEUKIN | PROLEUKIN | BLA103293 | TYPE 1 | 1992-05-05 |
| PREDNISONE | METICORTEN | NDA009766 | LABELING | 1992-07-06 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | EFFICACY | 1992-07-13 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1992-07-31 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 1992-07-31 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA011685 | MANUF (CMC) | 1992-11-05 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | MANUF (CMC) | 1992-11-05 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | MANUF (CMC) | 1992-12-08 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 1992-12-16 |
| BETAMETHASONE | CELESTONE | NDA012657 | LABELING | 1992-12-17 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | LABELING | 1992-12-17 |
| PREDNISONE | METICORTEN | NDA009766 | LABELING | 1992-12-17 |
| PACLITAXEL | TAXOL | NULL | TYPE 1 | 1992-12-29 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | MANUF (CMC) | 1993-01-28 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1993-03-04 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1993-03-09 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | EFFICACY | 1993-05-14 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1993-06-25 |
| PACLITAXEL | TAXOL | NULL | LABELING | 1993-07-23 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1993-08-05 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 1993-08-25 |
| CORTISONE ACETATE | CORTONE | NDA007110 | LABELING | 1993-08-26 |
| DEXAMETHASONE SODIUM PHOSPHATE | DECADRON | NDA012071 | LABELING | 1993-08-26 |
| CORTISONE ACETATE | CORTONE | NDA007750 | LABELING | 1993-08-27 |
| DEXAMETHASONE | DECADRON | NDA012376 | LABELING | 1993-08-27 |
| TRIAMCINOLONE | ARISTOCORT | NDA011161 | LABELING | 1993-09-07 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA011685 | LABELING | 1993-09-07 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | LABELING | 1993-09-07 |
| BETAMETHASONE | CELESTONE | NDA012657 | LABELING | 1993-09-07 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | LABELING | 1993-09-07 |
| PREDNISONE | METICORTEN | NDA009766 | LABELING | 1993-09-07 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 1993-09-07 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1993-11-05 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | MANUF (CMC) | 1993-11-08 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | LABELING | 1993-11-22 |
| HYDROCORTISONE | CORTEF | NDA008697 | MANUF (CMC) | 1993-12-08 |
| HYDROCORTISONE | CORTEF | NDA008697 | LABELING | 1993-12-28 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | MANUF (CMC) | 1993-12-28 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | LABELING | 1993-12-28 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | LABELING | 1993-12-28 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | MANUF (CMC) | 1994-01-07 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 1994-03-14 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1994-03-16 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 1994-03-25 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1994-03-25 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 1994-03-28 |
| TRIAMCINOLONE | ARISTOCORT | NDA011161 | MANUF (CMC) | 1994-03-30 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1994-04-06 |
| BETAMETHASONE | CELESTONE | NDA012657 | LABELING | 1994-04-13 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | LABELING | 1994-04-13 |
| PREDNISONE | METICORTEN | NDA009766 | LABELING | 1994-04-13 |
| PACLITAXEL | TAXOL | NULL | EFFICACY | 1994-04-13 |
| CORTISONE ACETATE | CORTONE | NDA007110 | MANUF (CMC) | 1994-04-20 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1994-04-21 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1994-04-22 |
| PACLITAXEL | TAXOL | NULL | EFFICACY | 1994-06-22 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1994-07-22 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA011685 | MANUF (CMC) | 1994-08-04 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | MANUF (CMC) | 1994-08-04 |
| BETAMETHASONE | CELESTONE | NDA012657 | MANUF (CMC) | 1994-08-10 |
| CORTISONE ACETATE | CORTONE | NDA007750 | MANUF (CMC) | 1994-08-18 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 1994-09-15 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1994-09-15 |
| TRIAMCINOLONE | ARISTOCORT | NDA011161 | MANUF (CMC) | 1994-10-18 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1994-10-19 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | MANUF (CMC) | 1994-10-20 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1994-11-29 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 1994-12-06 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 1995-01-10 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | MANUF (CMC) | 1995-01-12 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | MANUF (CMC) | 1995-01-24 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1995-02-15 |
| FLUOROURACIL | EFUDEX | NDA016831 | LABELING | 1995-03-21 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | MANUF (CMC) | 1995-03-22 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | MANUF (CMC) | 1995-04-10 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1995-04-25 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1995-04-26 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 1995-05-03 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1995-05-05 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | MANUF (CMC) | 1995-05-10 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | MANUF (CMC) | 1995-07-05 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1995-09-05 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | MANUF (CMC) | 1995-11-06 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA011685 | MANUF (CMC) | 1995-11-09 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | MANUF (CMC) | 1995-11-09 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | TYPE 3 | 1995-11-17 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | EFFICACY | 1995-12-05 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | MANUF (CMC) | 1995-12-13 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1995-12-20 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 1995-12-20 |
| METHOXSALEN | 8-MOP | NDA009048 | MANUF (CMC) | 1996-01-25 |
| CORTISONE ACETATE | CORTONE | NDA007750 | MANUF (CMC) | 1996-03-01 |
| CORTISONE ACETATE | CORTONE | NDA007110 | MANUF (CMC) | 1996-03-01 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 1996-03-01 |
| DEXAMETHASONE SODIUM PHOSPHATE | DECADRON | NDA012071 | MANUF (CMC) | 1996-03-01 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | LABELING | 1996-03-08 |
| PACLITAXEL | TAXOL | NULL | LABELING | 1996-04-01 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1996-04-03 |
| DAUNORUBICIN CITRATE | DAUNOXOME | NDA050704 | TYPE 3 | 1996-04-08 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1996-05-03 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 1996-05-22 |
| DACARBAZINE | DTIC-DOME | NDA017575 | MANUF (CMC) | 1996-05-24 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | MANUF (CMC) | 1996-07-10 |
| DAUNORUBICIN CITRATE | DAUNOXOME | NDA050704 | MANUF (CMC) | 1996-08-27 |
| BETAMETHASONE | CELESTONE | NDA012657 | MANUF (CMC) | 1996-09-10 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 1996-09-24 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 1996-09-24 |
| DAUNORUBICIN CITRATE | DAUNOXOME | NDA050704 | MANUF (CMC) | 1996-10-11 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1996-10-15 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | MANUF (CMC) | 1996-10-21 |
| DACARBAZINE | DTIC-DOME | NDA017575 | MANUF (CMC) | 1996-11-07 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 1996-11-13 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1996-11-25 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1996-12-13 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 1996-12-20 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1997-02-18 |
| DAUNORUBICIN CITRATE | DAUNOXOME | NDA050704 | MANUF (CMC) | 1997-02-24 |
| IMIQUIMOD | ALDARA | NDA020723 | TYPE 1 | 1997-02-27 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1997-03-05 |
| DAUNORUBICIN CITRATE | DAUNOXOME | NDA050704 | MANUF (CMC) | 1997-03-11 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | EFFICACY | 1997-03-26 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1997-04-07 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1997-04-25 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | S | 1997-04-29 |
| BETAMETHASONE | CELESTONE | NDA012657 | MANUF (CMC) | 1997-05-07 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 1997-05-20 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | LABELING | 1997-05-20 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1997-05-23 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | MANUF (CMC) | 1997-05-29 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | MANUF (CMC) | 1997-06-30 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 1997-07-21 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | LABELING | 1997-07-31 |
| PACLITAXEL | TAXOL | NULL | EFFICACY | 1997-08-04 |
| PREDNISONE | METICORTEN | NDA009766 | MANUF (CMC) | 1997-08-26 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | MANUF (CMC) | 1997-09-02 |
| DEXAMETHASONE SODIUM PHOSPHATE | DECADRON | NDA012071 | MANUF (CMC) | 1997-09-09 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1997-09-18 |
| METHOXSALEN | 8-MOP | NDA009048 | MANUF (CMC) | 1997-09-23 |
| METHOXSALEN | 8-MOP | NDA009048 | MANUF (CMC) | 1997-09-23 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 1997-10-14 |
| DEXAMETHASONE SODIUM PHOSPHATE | DECADRON | NDA012071 | MANUF (CMC) | 1997-10-22 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | EFFICACY | 1997-11-06 |
| IMIQUIMOD | ALDARA | NDA020723 | MANUF (CMC) | 1997-11-07 |
| IMIQUIMOD | ALDARA | NDA020723 | MANUF (CMC) | 1997-12-08 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1997-12-19 |
| ALDESLEUKIN | PROLEUKIN | BLA103293 | EFFICACY | 1998-01-09 |
| ALDESLEUKIN | PROLEUKIN | BLA103293 | EFFICACY | 1998-01-09 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | LABELING | 1998-01-21 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1998-02-13 |
| IMIQUIMOD | ALDARA | NDA020723 | MANUF (CMC) | 1998-02-20 |
| HYDROXYUREA | DROXIA | NDA016295 | EFFICACY | 1998-02-25 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1998-02-25 |
| DAUNORUBICIN CITRATE | DAUNOXOME | NDA050704 | MANUF (CMC) | 1998-02-26 |
| DAUNORUBICIN CITRATE | DAUNOXOME | NDA050704 | MANUF (CMC) | 1998-02-26 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 1998-02-26 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1998-02-26 |
| BETAMETHASONE | CELESTONE | NDA012657 | MANUF (CMC) | 1998-03-16 |
| METHOXSALEN | 8-MOP | NDA009048 | MANUF (CMC) | 1998-03-16 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | MANUF (CMC) | 1998-03-31 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1998-04-01 |
| METHOXSALEN | 8-MOP | NDA009048 | MANUF (CMC) | 1998-04-07 |
| PACLITAXEL | TAXOL | NULL | EFFICACY | 1998-04-09 |
| PACLITAXEL | TAXOL | NULL | LABELING | 1998-04-09 |
| PACLITAXEL | TAXOL | NULL | LABELING | 1998-04-09 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 1998-05-14 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 1998-06-18 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1998-06-22 |
| PACLITAXEL | TAXOL | NULL | EFFICACY | 1998-06-30 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | EFFICACY | 1998-08-18 |
| DACARBAZINE | DTIC-DOME | NDA017575 | MANUF (CMC) | 1998-09-24 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | MANUF (CMC) | 1998-10-16 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | MANUF (CMC) | 1998-10-16 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 1998-11-02 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1998-11-05 |
| HYDROXYUREA | DROXIA | NDA016295 | MANUF (CMC) | 1998-11-10 |
| BETAMETHASONE | CELESTONE | NDA012657 | MANUF (CMC) | 1998-11-19 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | MANUF (CMC) | 1998-11-30 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | MANUF (CMC) | 1998-12-01 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | LABELING | 1998-12-17 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | LABELING | 1998-12-17 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | LABELING | 1998-12-17 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | EFFICACY | 1998-12-17 |
| PACLITAXEL | TAXOL | NULL | LABELING | 1999-01-08 |
| PACLITAXEL | TAXOL | NULL | LABELING | 1999-01-08 |
| PACLITAXEL | TAXOL | NULL | LABELING | 1999-01-08 |
| PACLITAXEL | TAXOL | NULL | LABELING | 1999-01-08 |
| ALITRETINOIN | PANRETIN | NDA020886 | TYPE 1 | 1999-02-02 |
| DENILEUKIN DIFTITOX | ONTAK | BLA103767 | TYPE 1 | 1999-02-05 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 1999-02-12 |
| DEXAMETHASONE SODIUM PHOSPHATE | DECADRON | NDA012071 | MANUF (CMC) | 1999-02-17 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 1999-02-19 |
| METHOXSALEN | UVADEX | NDA020969 | TYPE 3 | 1999-02-25 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 1999-03-04 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | MANUF (CMC) | 1999-03-04 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 1999-03-16 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 1999-03-19 |
| DAUNORUBICIN CITRATE | DAUNOXOME | NDA050704 | MANUF (CMC) | 1999-03-23 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 1999-05-28 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 1999-05-28 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | EFFICACY | 1999-06-28 |
| ALITRETINOIN | PANRETIN | NDA020886 | MANUF (CMC) | 1999-07-12 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 1999-07-15 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 1999-08-11 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 1999-08-20 |
| TRIAMCINOLONE | ARISTOCORT | NDA011161 | MANUF (CMC) | 1999-09-17 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 1999-10-08 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA011685 | MANUF (CMC) | 1999-10-25 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | MANUF (CMC) | 1999-10-25 |
| PACLITAXEL | TAXOL | NULL | EFFICACY | 1999-10-25 |
| DAUNORUBICIN CITRATE | DAUNOXOME | NDA050704 | MANUF (CMC) | 1999-10-28 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 1999-10-29 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | LABELING | 1999-10-29 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 1999-11-25 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | MANUF (CMC) | 1999-12-01 |
| AMINOLEVULINIC ACID HYDROCHLORIDE | LEVULAN | NDA020965 | TYPE 1 | 1999-12-03 |
| PACLITAXEL | TAXOL | NULL | LABELING | 1999-12-10 |
| BEXAROTENE | TARGRETIN | NDA021055 | TYPE 1 | 1999-12-29 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2000-01-12 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 2000-02-09 |
| DEXAMETHASONE SODIUM PHOSPHATE | DECADRON | NDA012071 | MANUF (CMC) | 2000-02-18 |
| IMIQUIMOD | ALDARA | NDA020723 | MANUF (CMC) | 2000-03-07 |
| IMIQUIMOD | ALDARA | NDA020723 | MANUF (CMC) | 2000-03-07 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | LABELING | 2000-03-15 |
| DAUNORUBICIN CITRATE | DAUNOXOME | NDA050704 | MANUF (CMC) | 2000-03-28 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | MANUF (CMC) | 2000-03-30 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 2000-04-03 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | MANUF (CMC) | 2000-04-04 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 2000-04-04 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2000-04-05 |
| METHOXSALEN | 8-MOP | NDA009048 | MANUF (CMC) | 2000-04-05 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 2000-04-10 |
| DAUNORUBICIN CITRATE | DAUNOXOME | NDA050704 | MANUF (CMC) | 2000-05-23 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | MANUF (CMC) | 2000-06-13 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 2000-06-15 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 2000-06-15 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2000-06-20 |
| PACLITAXEL | TAXOL | NULL | EFFICACY | 2000-06-20 |
| ALDESLEUKIN | PROLEUKIN | BLA103293 | LABELING | 2000-06-27 |
| BEXAROTENE | TARGRETIN | NDA021056 | TYPE 3 | 2000-06-28 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2000-07-07 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2000-07-07 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 2000-08-24 |
| IMIQUIMOD | ALDARA | NDA020723 | MANUF (CMC) | 2000-09-27 |
| DICLOFENAC SODIUM | SOLARAZE | NULL | TYPE 3 | 2000-10-16 |
| FLUOROURACIL | CARAC | NDA020985 | TYPE 3 | 2000-10-27 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 2000-11-02 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 2000-11-08 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | LABELING | 2000-11-08 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | MANUF (CMC) | 2000-11-17 |
| ALITRETINOIN | PANRETIN | NDA020886 | MANUF (CMC) | 2000-11-21 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 2001-01-05 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | TYPE 1 | 2001-01-19 |
| FLUOROURACIL | CARAC | NDA020985 | LABELING | 2001-01-29 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 2001-01-30 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 2001-01-30 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2001-02-12 |
| BEXAROTENE | TARGRETIN | NDA021055 | MANUF (CMC) | 2001-02-14 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2001-02-20 |
| HYDROCORTISONE | CORTEF | NDA008697 | MANUF (CMC) | 2001-03-16 |
| IMIQUIMOD | ALDARA | NDA020723 | MANUF (CMC) | 2001-03-28 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2001-04-04 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 2001-04-10 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 2001-05-08 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | LABELING | 2001-05-08 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 2001-05-23 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | EFFICACY | 2001-06-21 |
| FLUOROURACIL | CARAC | NDA020985 | MANUF (CMC) | 2001-07-02 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2001-07-03 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2001-07-03 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 2001-07-24 |
| AMINOLEVULINIC ACID HYDROCHLORIDE | LEVULAN | NDA020965 | MANUF (CMC) | 2001-07-27 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | EFFICACY | 2001-08-07 |
| DICLOFENAC SODIUM | SOLARAZE | NULL | LABELING | 2001-08-17 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA011685 | MANUF (CMC) | 2001-08-29 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | MANUF (CMC) | 2001-08-29 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2001-08-30 |
| DICLOFENAC SODIUM | SOLARAZE | NULL | MANUF (CMC) | 2001-10-02 |
| METHOXSALEN | 8-MOP | NDA009048 | MANUF (CMC) | 2001-10-31 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2001-11-29 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2001-11-29 |
| IMIQUIMOD | ALDARA | NDA020723 | LABELING | 2001-12-08 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | MANUF (CMC) | 2002-01-03 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 2002-01-10 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | LABELING | 2002-01-10 |
| BEXAROTENE | TARGRETIN | NDA021055 | MANUF (CMC) | 2002-01-29 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2002-02-01 |
| DAUNORUBICIN CITRATE | DAUNOXOME | NDA050704 | MANUF (CMC) | 2002-02-10 |
| DICLOFENAC SODIUM | SOLARAZE | NULL | MANUF (CMC) | 2002-02-19 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 2002-02-20 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 2002-02-20 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | LABELING | 2002-02-20 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | LABELING | 2002-02-20 |
| DAUNORUBICIN CITRATE | DAUNOXOME | NDA050704 | LABELING | 2002-02-21 |
| PACLITAXEL | TAXOL | NULL | LABELING | 2002-03-04 |
| PACLITAXEL | TAXOL | NULL | LABELING | 2002-03-04 |
| BEXAROTENE | TARGRETIN | NDA021056 | MANUF (CMC) | 2002-03-14 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 2002-04-02 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | MANUF (CMC) | 2002-04-18 |
| DICLOFENAC SODIUM | SOLARAZE | NULL | MANUF (CMC) | 2002-05-20 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | MANUF (CMC) | 2002-05-22 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | MANUF (CMC) | 2002-06-18 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2002-06-21 |
| DEXAMETHASONE | DECADRON | NDA011664 | MANUF (CMC) | 2002-06-25 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2002-07-17 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | MANUF (CMC) | 2002-08-09 |
| PACLITAXEL | TAXOL | NULL | LABELING | 2002-08-29 |
| IMIQUIMOD | ALDARA | NDA020723 | LABELING | 2002-09-03 |
| IMIQUIMOD | ALDARA | NDA020723 | LABELING | 2002-09-03 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 2002-09-25 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 2002-09-25 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 2002-10-21 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 2002-10-21 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | MANUF (CMC) | 2002-10-25 |
| PREDNISONE | METICORTEN | NDA009766 | LABELING | 2002-11-12 |
| PREDNISONE | METICORTEN | NDA009766 | LABELING | 2002-11-12 |
| PREDNISONE | METICORTEN | NDA009766 | LABELING | 2002-11-12 |
| FLUOROURACIL | CARAC | NDA020985 | MANUF (CMC) | 2002-11-15 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2002-11-21 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2002-11-27 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 2002-12-12 |
| DICLOFENAC SODIUM | SOLARAZE | NULL | MANUF (CMC) | 2002-12-13 |
| IMIQUIMOD | ALDARA | NDA020723 | LABELING | 2002-12-15 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | MANUF (CMC) | 2002-12-17 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 2003-01-03 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | LABELING | 2003-01-17 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | LABELING | 2003-01-17 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 2003-02-27 |
| PACLITAXEL | TAXOL | NULL | LABELING | 2003-03-05 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2003-03-14 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | LABELING | 2003-03-19 |
| AMINOLEVULINIC ACID HYDROCHLORIDE | LEVULAN | NDA020965 | LABELING | 2003-03-26 |
| METHOXSALEN | 8-MOP | NDA009048 | LABELING | 2003-03-26 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | TYPE 3 | 2003-04-18 |
| BEXAROTENE | TARGRETIN | NDA021055 | LABELING | 2003-04-21 |
| BEXAROTENE | TARGRETIN | NDA021055 | LABELING | 2003-04-21 |
| DICLOFENAC SODIUM | SOLARAZE | NULL | LABELING | 2003-04-29 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | EFFICACY | 2003-05-08 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 2003-05-08 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2003-05-20 |
| HYDROXYUREA | DROXIA | NDA016295 | EFFICACY | 2003-06-26 |
| AMINOLEVULINIC ACID HYDROCHLORIDE | LEVULAN | NDA020965 | LABELING | 2003-06-27 |
| AMINOLEVULINIC ACID HYDROCHLORIDE | LEVULAN | NDA020965 | MANUF (CMC) | 2003-07-11 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 2003-07-29 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | LABELING | 2003-10-24 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 2003-10-24 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 2003-11-07 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2003-11-26 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2003-12-08 |
| FLUOROURACIL | CARAC | NDA020985 | LABELING | 2003-12-16 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 2004-01-27 |
| MECHLORETHAMINE HYDROCHLORIDE | MUSTARGEN | NDA006695 | LABELING | 2004-02-09 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2004-02-19 |
| IMIQUIMOD | ALDARA | NDA020723 | EFFICACY | 2004-03-02 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2004-03-05 |
| DICLOFENAC SODIUM | SOLARAZE | NULL | MANUF (CMC) | 2004-03-18 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2004-04-27 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 2004-05-17 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2004-06-23 |
| FLUOROURACIL | EFUDEX | NDA016831 | LABELING | 2004-06-24 |
| IMIQUIMOD | ALDARA | NDA020723 | EFFICACY | 2004-07-14 |
| DICLOFENAC SODIUM | SOLARAZE | NULL | MANUF (CMC) | 2004-07-23 |
| METHYL AMINOLEVULINATE HYDROCHLORIDE | METVIXIA | NDA021415 | TYPE 3 | 2004-07-27 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2004-09-23 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2004-10-01 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2004-10-15 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2004-10-15 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | EFFICACY | 2004-10-27 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | S | 2004-10-27 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | LABELING | 2004-12-03 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 2004-12-15 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | EFFICACY | 2005-01-28 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2005-03-14 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 2005-04-13 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2005-05-04 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2005-05-18 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | MANUF (CMC) | 2005-05-19 |
| METHYL AMINOLEVULINATE HYDROCHLORIDE | METVIXIA | NDA021415 | LABELING | 2005-05-31 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2005-07-22 |
| IMIQUIMOD | ALDARA | NDA020723 | LABELING | 2005-08-09 |
| FLUOROURACIL | EFUDEX | NDA016831 | LABELING | 2005-10-13 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2005-10-20 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2005-11-03 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2006-05-10 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2006-05-31 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | LABELING | 2006-05-31 |
| PREDNISOLONE SODIUM PHOSPHATE | ORAPRED ODT | NDA021959 | TYPE 3 | 2006-06-01 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | LABELING | 2006-07-12 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2006-07-28 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2006-09-19 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2006-09-27 |
| VORINOSTAT | ZOLINZA | NDA021991 | TYPE 1 | 2006-10-06 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2006-10-10 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2006-10-19 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2006-10-19 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2006-10-19 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2006-10-19 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2006-10-19 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2006-10-30 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | LABELING | 2006-11-20 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2006-12-22 |
| IMIQUIMOD | ALDARA | NDA020723 | EFFICACY | 2007-03-22 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | LABELING | 2007-05-17 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | EFFICACY | 2007-05-17 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | S | 2007-05-22 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2007-05-22 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2007-05-31 |
| DICLOFENAC SODIUM | SOLARAZE | NULL | MANUF (CMC) | 2007-06-01 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2007-07-13 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2007-09-13 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 2007-12-07 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 2007-12-07 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 2007-12-07 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 2007-12-07 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 2007-12-07 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 2007-12-07 |
| VINBLASTINE SULFATE | VELBAN | NDA012665 | LABELING | 2007-12-07 |
| PREDNISOLONE ACETATE | FLO-PRED | NDA022067 | TYPE 3 | 2008-01-17 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | EFFICACY | 2008-03-26 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | MANUF (CMC) | 2008-04-30 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2008-05-02 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | EFFICACY | 2008-06-10 |
| TRIAMCINOLONE ACETONIDE | TRIVARIS | NDA022220 | TYPE 3 | 2008-06-16 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | LABELING | 2008-06-24 |
| METHYL AMINOLEVULINATE HYDROCHLORIDE | METVIXIA | NDA021415 | EFFICACY | 2008-06-26 |
| PREDNISOLONE ACETATE | FLO-PRED | NDA022067 | MANUF (CMC) | 2008-06-27 |
| VORINOSTAT | ZOLINZA | NDA021991 | LABELING | 2008-07-22 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2008-09-26 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2008-09-26 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2008-09-26 |
| PREDNISOLONE ACETATE | FLO-PRED | NDA022067 | MANUF (CMC) | 2008-10-03 |
| DENILEUKIN DIFTITOX | ONTAK | BLA103767 | EFFICACY | 2008-10-15 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | EFFICACY | 2008-12-11 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | S | 2008-12-11 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2008-12-19 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2009-02-10 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | EFFICACY | 2009-03-10 |
| TRIAMCINOLONE | ARISTOCORT | NDA011161 | LABELING | 2009-03-13 |
| TRIAMCINOLONE | ARISTOCORT | NDA011161 | LABELING | 2009-03-13 |
| TRIAMCINOLONE | ARISTOCORT | NDA011161 | LABELING | 2009-03-13 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | LABELING | 2009-04-07 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | LABELING | 2009-04-07 |
| ALDESLEUKIN | PROLEUKIN | BLA103293 | LABELING | 2009-04-08 |
| PREDNISOLONE SODIUM PHOSPHATE | ORAPRED ODT | NDA021959 | MANUF (CMC) | 2009-04-27 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | EFFICACY | 2009-05-08 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2009-05-08 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2009-05-27 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2009-05-27 |
| AMINOLEVULINIC ACID HYDROCHLORIDE | LEVULAN | NDA020965 | LABELING | 2009-07-17 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2009-08-07 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2009-08-07 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | LABELING | 2009-09-01 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | LABELING | 2009-09-01 |
| VORINOSTAT | ZOLINZA | NDA021991 | EFFICACY | 2009-09-23 |
| ROMIDEPSIN | ISTODAX | NDA022393 | TYPE 1 | 2009-11-05 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | LABELING | 2010-01-14 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2010-01-26 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | LABELING | 2010-01-28 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2010-02-16 |
| DENILEUKIN DIFTITOX | ONTAK | BLA103767 | LABELING | 2010-03-05 |
| AMINOLEVULINIC ACID HYDROCHLORIDE | LEVULAN | NDA020965 | EFFICACY | 2010-03-12 |
| ALDESLEUKIN | PROLEUKIN | BLA103293 | LABELING | 2010-03-17 |
| IMIQUIMOD | ZYCLARA | NDA022483 | TYPE 5 | 2010-03-25 |
| PREDNISOLONE ACETATE | FLO-PRED | NDA022067 | MANUF (CMC) | 2010-05-06 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2010-05-07 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 2010-06-16 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | LABELING | 2010-06-24 |
| ALDESLEUKIN | PROLEUKIN | BLA103293 | LABELING | 2010-07-06 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2010-07-21 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2010-07-21 |
| PREDNISOLONE SODIUM PHOSPHATE | ORAPRED ODT | NDA021959 | LABELING | 2010-07-28 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 2010-07-30 |
| PACLITAXEL | TAXOL | NULL | LABELING | 2010-08-13 |
| IMIQUIMOD | ALDARA | NDA020723 | LABELING | 2010-10-14 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | S | 2011-02-01 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2011-02-01 |
| IMIQUIMOD | ZYCLARA | NDA022483 | EFFICACY | 2011-03-24 |
| IPILIMUMAB | YERVOY | BLA125377 | TYPE 1 | 2011-03-25 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | EFFICACY | 2011-03-29 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2011-04-01 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2011-04-01 |
| BEXAROTENE | TARGRETIN | NDA021056 | LABELING | 2011-04-08 |
| ROMIDEPSIN | ISTODAX | NDA022393 | LABELING | 2011-04-12 |
| PACLITAXEL | TAXOL | NULL | LABELING | 2011-05-02 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2011-05-06 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2011-05-13 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2011-05-13 |
| BEXAROTENE | TARGRETIN | NDA021055 | LABELING | 2011-05-16 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2011-06-06 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2011-06-06 |
| VORINOSTAT | ZOLINZA | NDA021991 | LABELING | 2011-06-10 |
| PREDNISOLONE ACETATE | FLO-PRED | NDA022067 | LABELING | 2011-06-14 |
| ROMIDEPSIN | ISTODAX | NDA022393 | EFFICACY | 2011-06-16 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | LABELING | 2011-06-16 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | LABELING | 2011-07-15 |
| IMIQUIMOD | ZYCLARA | NDA022483 | EFFICACY | 2011-07-15 |
| ALDESLEUKIN | PROLEUKIN | BLA103293 | LABELING | 2011-07-19 |
| IPILIMUMAB | YERVOY | BLA125377 | LABELING | 2011-07-28 |
| VEMURAFENIB | ZELBORAF | NDA202429 | TYPE 1 | 2011-08-17 |
| BRENTUXIMAB VEDOTIN | ADCETRIS | BLA125388 | TYPE 1 | 2011-08-19 |
| BRENTUXIMAB VEDOTIN | ADCETRIS | BLA125388 | EFFICACY | 2011-08-19 |
| DENILEUKIN DIFTITOX | ONTAK | BLA103767 | S | 2011-08-30 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 2011-09-26 |
| IMIQUIMOD | ZYCLARA | NDA022483 | LABELING | 2011-09-29 |
| ROMIDEPSIN | ISTODAX | NDA022393 | LABELING | 2011-09-30 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 2011-10-20 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | LABELING | 2011-10-20 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 2011-11-01 |
| VORINOSTAT | ZOLINZA | NDA021991 | LABELING | 2011-11-14 |
| DICLOFENAC SODIUM | SOLARAZE | NULL | LABELING | 2011-12-08 |
| ALDESLEUKIN | PROLEUKIN | BLA103293 | LABELING | 2011-12-16 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2011-12-20 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2011-12-22 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2011-12-22 |
| BEXAROTENE | TARGRETIN | NDA021055 | LABELING | 2012-01-06 |
| BRENTUXIMAB VEDOTIN | ADCETRIS | BLA125388 | LABELING | 2012-01-13 |
| BRENTUXIMAB VEDOTIN | ADCETRIS | BLA125388 | LABELING | 2012-01-13 |
| INGENOL MEBUTATE | PICATO | NULL | TYPE 1 | 2012-01-23 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2012-01-26 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2012-01-26 |
| VISMODEGIB | ERIVEDGE | NDA203388 | TYPE 1 | 2012-01-30 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2012-01-31 |
| IMIQUIMOD | ZYCLARA | NDA022483 | MANUF (CMC) | 2012-02-14 |
| IPILIMUMAB | YERVOY | BLA125377 | LABELING | 2012-02-16 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 2012-03-31 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 2012-03-31 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2012-06-04 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2012-06-04 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2012-07-02 |
| ALDESLEUKIN | PROLEUKIN | BLA103293 | LABELING | 2012-07-30 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | LABELING | 2012-09-19 |
| IPILIMUMAB | YERVOY | BLA125377 | LABELING | 2012-10-26 |
| METHYL AMINOLEVULINATE HYDROCHLORIDE | METVIXIA | NDA021415 | LABELING | 2012-11-20 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2012-12-26 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 2013-01-09 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2013-01-25 |
| POMALIDOMIDE | POMALYST | NDA204026 | TYPE 1 | 2013-02-08 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2013-02-21 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 2013-03-13 |
| METHOXSALEN | 8-MOP | NDA009048 | MANUF (CMC) | 2013-03-22 |
| VORINOSTAT | ZOLINZA | NDA021991 | LABELING | 2013-04-24 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | LABELING | 2013-05-07 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | LABELING | 2013-05-07 |
| VISMODEGIB | ERIVEDGE | NDA203388 | MANUF (CMC) | 2013-05-08 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2013-05-15 |
| IPILIMUMAB | YERVOY | BLA125377 | LABELING | 2013-05-22 |
| TRAMETINIB DIMETHYL SULFOXIDE | MEKINIST | NDA204114 | TYPE 1 | 2013-05-29 |
| DABRAFENIB MESYLATE | TAFINLAR | NDA202806 | TYPE 1 | 2013-05-29 |
| VISMODEGIB | ERIVEDGE | NDA203388 | MANUF (CMC) | 2013-06-03 |
| METHOXSALEN | UVADEX | NDA020969 | MANUF (CMC) | 2013-06-05 |
| ROMIDEPSIN | ISTODAX | NDA022393 | LABELING | 2013-06-13 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2013-07-02 |
| VEMURAFENIB | ZELBORAF | NDA202429 | LABELING | 2013-07-03 |
| VEMURAFENIB | ZELBORAF | NDA202429 | LABELING | 2013-07-03 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | MANUF (CMC) | 2013-07-18 |
| BEXAROTENE | TARGRETIN | NDA021056 | MANUF (CMC) | 2013-07-22 |
| BRENTUXIMAB VEDOTIN | ADCETRIS | BLA125388 | LABELING | 2013-08-19 |
| MECHLORETHAMINE HYDROCHLORIDE | VALCHLOR | NDA202317 | TYPE 5 | 2013-08-23 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | LABELING | 2013-08-30 |
| ROMIDEPSIN | ISTODAX | NDA022393 | MANUF (CMC) | 2013-09-13 |
| METHOXSALEN | 8-MOP | NDA009048 | LABELING | 2013-09-20 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 2013-09-27 |
| POMALIDOMIDE | POMALYST | NDA204026 | MANUF (CMC) | 2013-10-03 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | MANUF (CMC) | 2013-10-07 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | LABELING | 2013-10-08 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 2013-10-10 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | MANUF (CMC) | 2013-10-11 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2013-10-30 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 2013-10-31 |
| METHOXSALEN | UVADEX | NDA020969 | LABELING | 2013-11-01 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 2013-11-08 |
| AMINOLEVULINIC ACID HYDROCHLORIDE | LEVULAN | NDA020965 | MANUF (CMC) | 2013-11-14 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2013-11-15 |
| POMALIDOMIDE | POMALYST | NDA204026 | REMS | 2013-11-15 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2013-11-15 |
| ALITRETINOIN | PANRETIN | NDA020886 | MANUF (CMC) | 2013-11-22 |
| IPILIMUMAB | YERVOY | BLA125377 | LABELING | 2013-12-05 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2013-12-09 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | MANUF (CMC) | 2013-12-12 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2013-12-12 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | EFFICACY | 2013-12-18 |
| VISMODEGIB | ERIVEDGE | NDA203388 | MANUF (CMC) | 2013-12-19 |
| DABRAFENIB MESYLATE | TAFINLAR | NDA202806 | LABELING | 2013-12-26 |
| TRAMETINIB DIMETHYL SULFOXIDE | MEKINIST | NDA204114 | EFFICACY | 2014-01-08 |
| DABRAFENIB MESYLATE | TAFINLAR | NDA202806 | EFFICACY | 2014-01-09 |
| VEMURAFENIB | ZELBORAF | NDA202429 | LABELING | 2014-02-06 |
| PREDNISOLONE ACETATE | FLO-PRED | NDA022067 | MANUF (CMC) | 2014-02-28 |
| INGENOL MEBUTATE | PICATO | NULL | LABELING | 2014-03-12 |
| POMALIDOMIDE | POMALYST | NDA204026 | LABELING | 2014-03-13 |
| VEMURAFENIB | ZELBORAF | NDA202429 | EFFICACY | 2014-03-19 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 2014-03-28 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 2014-03-28 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2014-04-02 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 2014-04-04 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 2014-04-04 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | MANUF (CMC) | 2014-04-14 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | MANUF (CMC) | 2014-05-21 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2014-05-22 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | MANUF (CMC) | 2014-06-09 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 2014-06-09 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 2014-06-09 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 2014-06-24 |
| PACLITAXEL | TAXOL | NULL | MANUF (CMC) | 2014-06-27 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA011685 | LABELING | 2014-07-03 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | LABELING | 2014-07-03 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | LABELING | 2014-07-03 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | LABELING | 2014-07-03 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | LABELING | 2014-07-03 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | LABELING | 2014-07-03 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | LABELING | 2014-07-03 |
| MECHLORETHAMINE HYDROCHLORIDE | VALCHLOR | NDA202317 | MANUF (CMC) | 2014-07-08 |
| MECHLORETHAMINE HYDROCHLORIDE | VALCHLOR | NDA202317 | LABELING | 2014-07-09 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 2014-07-22 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 2014-07-22 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 2014-07-22 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2014-07-31 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2014-08-11 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2014-08-11 |
| TRAMETINIB DIMETHYL SULFOXIDE | MEKINIST | NDA204114 | MANUF (CMC) | 2014-08-13 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2014-08-14 |
| DABRAFENIB MESYLATE | TAFINLAR | NDA202806 | MANUF (CMC) | 2014-08-25 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2014-08-30 |
| TRIAMCINOLONE DIACETATE | ARISTOCORT | NDA012802 | MANUF (CMC) | 2014-09-04 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | TYPE 1 | 2014-09-04 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 2014-09-06 |
| POMALIDOMIDE | POMALYST | NDA204026 | REMS | 2014-09-12 |
| VEMURAFENIB | ZELBORAF | NDA202429 | MANUF (CMC) | 2014-09-23 |
| TRAMETINIB DIMETHYL SULFOXIDE | MEKINIST | NDA204114 | MANUF (CMC) | 2014-09-26 |
| PREDNISOLONE ACETATE | FLO-PRED | NDA022067 | MANUF (CMC) | 2014-09-29 |
| PREDNISOLONE ACETATE | FLO-PRED | NDA022067 | MANUF (CMC) | 2014-10-03 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2014-10-09 |
| MECHLORETHAMINE HYDROCHLORIDE | VALCHLOR | NDA202317 | MANUF (CMC) | 2014-10-13 |
| VISMODEGIB | ERIVEDGE | NDA203388 | MANUF (CMC) | 2014-10-15 |
| ROMIDEPSIN | ISTODAX | NDA022393 | EFFICACY | 2014-10-15 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2014-11-17 |
| FLUOROURACIL | CARAC | NDA020985 | MANUF (CMC) | 2014-11-20 |
| BRENTUXIMAB VEDOTIN | ADCETRIS | BLA125388 | LABELING | 2014-11-23 |
| BRENTUXIMAB VEDOTIN | ADCETRIS | BLA125388 | LABELING | 2014-11-23 |
| IMIQUIMOD | ZYCLARA | NDA022483 | MANUF (CMC) | 2014-11-24 |
| VEMURAFENIB | ZELBORAF | NDA202429 | LABELING | 2014-11-25 |
| IMIQUIMOD | ALDARA | NDA020723 | MANUF (CMC) | 2014-11-26 |
| BEXAROTENE | TARGRETIN | NDA021055 | MANUF (CMC) | 2014-12-02 |
| NIVOLUMAB | OPDIVO | BLA125554 | TYPE 1 | 2014-12-22 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | MANUF (CMC) | 2015-01-22 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2015-01-30 |
| IMIQUIMOD | ALDARA | NDA020723 | MANUF (CMC) | 2015-02-03 |
| INGENOL MEBUTATE | PICATO | NULL | MANUF (CMC) | 2015-02-03 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | LABELING | 2015-02-04 |
| BEXAROTENE | TARGRETIN | NDA021056 | MANUF (CMC) | 2015-02-09 |
| PACLITAXEL | TAXOL | NULL | LABELING | 2015-03-03 |
| IPILIMUMAB | YERVOY | BLA125377 | REMS | 2015-03-16 |
| IPILIMUMAB | YERVOY | BLA125377 | LABELING | 2015-03-19 |
| DICLOFENAC SODIUM | SOLARAZE | NULL | MANUF (CMC) | 2015-03-30 |
| NIVOLUMAB | OPDIVO | BLA125554 | LABELING | 2015-04-14 |
| NIVOLUMAB | OPDIVO | BLA125554 | LABELING | 2015-04-14 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | EFFICACY | 2015-04-16 |
| POMALIDOMIDE | POMALYST | NDA204026 | LABELING | 2015-04-23 |
| POMALIDOMIDE | POMALYST | NDA204026 | EFFICACY | 2015-04-23 |
| POMALIDOMIDE | POMALYST | NDA204026 | LABELING | 2015-04-23 |
| FLUOROURACIL | CARAC | NDA020985 | MANUF (CMC) | 2015-04-29 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2015-05-06 |
| ALDESLEUKIN | PROLEUKIN | BLA103293 | LABELING | 2015-05-06 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2015-05-13 |
| VISMODEGIB | ERIVEDGE | NDA203388 | LABELING | 2015-05-21 |
| VISMODEGIB | ERIVEDGE | NDA203388 | LABELING | 2015-05-21 |
| VISMODEGIB | ERIVEDGE | NDA203388 | LABELING | 2015-05-21 |
| VISMODEGIB | ERIVEDGE | NDA203388 | LABELING | 2015-05-21 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2015-05-21 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2015-05-21 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | LABELING | 2015-06-19 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2015-07-16 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2015-07-16 |
| SONIDEGIB PHOSPHATE | ODOMZO | NDA205266 | TYPE 1 | 2015-07-24 |
| METHOXSALEN | 8-MOP | NDA009048 | MANUF (CMC) | 2015-07-28 |
| BEXAROTENE | TARGRETIN | NDA021055 | EFFICACY | 2015-07-29 |
| METHOXSALEN | 8-MOP | NDA009048 | MANUF (CMC) | 2015-07-30 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | MANUF (CMC) | 2015-08-06 |
| VEMURAFENIB | ZELBORAF | NDA202429 | LABELING | 2015-08-11 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 2015-08-14 |
| BRENTUXIMAB VEDOTIN | ADCETRIS | BLA125388 | EFFICACY | 2015-08-17 |
| BRENTUXIMAB VEDOTIN | ADCETRIS | BLA125388 | LABELING | 2015-08-17 |
| IPILIMUMAB | YERVOY | BLA125377 | LABELING | 2015-08-17 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2015-08-18 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | MANUF (CMC) | 2015-09-10 |
| POMALIDOMIDE | POMALYST | NDA204026 | REMS | 2015-09-14 |
| BEXAROTENE | TARGRETIN | NDA021055 | MANUF (CMC) | 2015-09-14 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2015-09-16 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 2015-09-23 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2015-09-30 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2015-10-02 |
| INGENOL MEBUTATE | PICATO | NULL | LABELING | 2015-10-06 |
| VORINOSTAT | ZOLINZA | NDA021991 | MANUF (CMC) | 2015-10-07 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | MANUF (CMC) | 2015-10-09 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2015-10-09 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 2015-10-26 |
| TALIMOGENE LAHERPAREPVEC | IMLYGIC | BLA125518 | TYPE 1 | 2015-10-27 |
| POMALIDOMIDE | POMALYST | NDA204026 | REMS | 2015-10-27 |
| IPILIMUMAB | YERVOY | BLA125377 | EFFICACY | 2015-10-28 |
| MECHLORETHAMINE HYDROCHLORIDE | VALCHLOR | NDA202317 | LABELING | 2015-10-29 |
| FLUOROURACIL | EFUDEX | NDA016831 | MANUF (CMC) | 2015-11-04 |
| COBIMETINIB FUMARATE | COTELLIC | NDA206192 | TYPE 1 | 2015-11-10 |
| INGENOL MEBUTATE | PICATO | NULL | EFFICACY | 2015-11-19 |
| TRAMETINIB DIMETHYL SULFOXIDE | MEKINIST | NDA204114 | EFFICACY | 2015-11-20 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 2015-11-20 |
| DABRAFENIB MESYLATE | TAFINLAR | NDA202806 | EFFICACY | 2015-11-20 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2015-11-23 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2015-11-23 |
| POMALIDOMIDE | POMALYST | NDA204026 | REMS | 2015-12-01 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 2015-12-02 |
| VORINOSTAT | ZOLINZA | NDA021991 | LABELING | 2015-12-17 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2015-12-18 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2015-12-18 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | MANUF (CMC) | 2015-12-28 |
| AMINOLEVULINIC ACID HYDROCHLORIDE | LEVULAN | NDA020965 | MANUF (CMC) | 2016-01-07 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2016-01-23 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2016-01-23 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | MANUF (CMC) | 2016-01-29 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | LABELING | 2016-01-29 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | MANUF (CMC) | 2016-02-03 |
| SONIDEGIB PHOSPHATE | ODOMZO | NDA205266 | LABELING | 2016-02-17 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | MANUF (CMC) | 2016-02-25 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | MANUF (CMC) | 2016-03-01 |
| BRENTUXIMAB VEDOTIN | ADCETRIS | BLA125388 | LABELING | 2016-03-04 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2016-03-23 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2016-03-23 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | MANUF (CMC) | 2016-03-29 |
| PREDNISOLONE SODIUM PHOSPHATE | ORAPRED ODT | NDA021959 | MANUF (CMC) | 2016-04-08 |
| MECHLORETHAMINE HYDROCHLORIDE | VALCHLOR | NDA202317 | MANUF (CMC) | 2016-04-08 |
| INGENOL MEBUTATE | PICATO | NULL | MANUF (CMC) | 2016-04-15 |
| POMALIDOMIDE | POMALYST | NDA204026 | REMS | 2016-04-22 |
| DICLOFENAC SODIUM | SOLARAZE | NULL | LABELING | 2016-05-09 |
| VEMURAFENIB | ZELBORAF | NDA202429 | LABELING | 2016-05-09 |
| AMINOLEVULINIC ACID HYDROCHLORIDE | AMELUZ | NDA208081 | TYPE 3 | 2016-05-10 |
| SONIDEGIB PHOSPHATE | ODOMZO | NDA205266 | LABELING | 2016-05-12 |
| DICLOFENAC SODIUM | SOLARAZE | NULL | MANUF (CMC) | 2016-05-16 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2016-05-17 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | TYPE 1 | 2016-05-18 |
| COBIMETINIB FUMARATE | COTELLIC | NDA206192 | LABELING | 2016-05-31 |
| DABRAFENIB MESYLATE | TAFINLAR | NDA202806 | LABELING | 2016-06-16 |
| POMALIDOMIDE | POMALYST | NDA204026 | LABELING | 2016-06-30 |
| POMALIDOMIDE | POMALYST | NDA204026 | LABELING | 2016-06-30 |
| FLUOROURACIL | FLUOROPLEX | NDA016988 | LABELING | 2016-07-25 |
| ROMIDEPSIN | ISTODAX | NDA022393 | LABELING | 2016-07-27 |
| PREDNISOLONE SODIUM PHOSPHATE | ORAPRED ODT | NDA021959 | MANUF (CMC) | 2016-07-28 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 2016-07-29 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2016-08-05 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | EFFICACY | 2016-08-25 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2016-08-25 |
| VEMURAFENIB | ZELBORAF | NDA202429 | EFFICACY | 2016-08-31 |
| HYDROCORTISONE | CORTEF | NDA008697 | LABELING | 2016-09-08 |
| HYDROCORTISONE | CORTEF | NDA008697 | LABELING | 2016-09-08 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | LABELING | 2016-09-08 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | LABELING | 2016-09-08 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | LABELING | 2016-09-08 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | LABELING | 2016-09-08 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | LABELING | 2016-09-08 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2016-09-13 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2016-09-13 |
| INGENOL MEBUTATE | PICATO | NULL | LABELING | 2016-09-13 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2016-09-27 |
| BRENTUXIMAB VEDOTIN | ADCETRIS | BLA125388 | LABELING | 2016-09-28 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2016-10-04 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2016-10-24 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2016-10-24 |
| CYCLOPHOSPHAMIDE | CYTOXAN (LYOPHILIZED) | NDA012142 | MANUF (CMC) | 2016-10-31 |
| VISMODEGIB | ERIVEDGE | NDA203388 | LABELING | 2016-11-02 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2016-11-10 |
| MECHLORETHAMINE HYDROCHLORIDE | VALCHLOR | NDA202317 | MANUF (CMC) | 2016-12-22 |
| INGENOL MEBUTATE | PICATO | NULL | MANUF (CMC) | 2017-01-26 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2017-02-02 |
| TRAMETINIB DIMETHYL SULFOXIDE | MEKINIST | NDA204114 | LABELING | 2017-02-24 |
| IPILIMUMAB | YERVOY | BLA125377 | LABELING | 2017-03-03 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2017-03-14 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | LABELING | 2017-03-15 |
| AVELUMAB | BAVENCIO | BLA761049 | TYPE 1 | 2017-03-23 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2017-04-17 |
| VEMURAFENIB | ZELBORAF | NDA202429 | LABELING | 2017-04-17 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2017-04-24 |
| AVELUMAB | BAVENCIO | BLA761049 | LABELING | 2017-04-25 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2017-04-25 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2017-05-10 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2017-05-17 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2017-05-18 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2017-05-18 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2017-05-23 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2017-05-25 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2017-06-12 |
| DABRAFENIB MESYLATE | TAFINLAR | NDA202806 | LABELING | 2017-06-16 |
| TRAMETINIB DIMETHYL SULFOXIDE | MEKINIST | NDA204114 | EFFICACY | 2017-06-22 |
| DABRAFENIB MESYLATE | TAFINLAR | NDA202806 | EFFICACY | 2017-06-22 |
| POMALIDOMIDE | POMALYST | NDA204026 | REMS | 2017-06-27 |
| IPILIMUMAB | YERVOY | BLA125377 | EFFICACY | 2017-07-21 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | LABELING | 2017-07-27 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | LABELING | 2017-07-27 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2017-07-31 |
| VISMODEGIB | ERIVEDGE | NDA203388 | LABELING | 2017-08-01 |
| AMINOLEVULINIC ACID HYDROCHLORIDE | AMELUZ | NDA208081 | LABELING | 2017-09-06 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2017-09-06 |
| NIVOLUMAB | OPDIVO | BLA125554 | LABELING | 2017-09-06 |
| VEMURAFENIB | ZELBORAF | NDA202429 | EFFICACY | 2017-09-13 |
| VEMURAFENIB | ZELBORAF | NDA202429 | LABELING | 2017-09-13 |
| SONIDEGIB PHOSPHATE | ODOMZO | NDA205266 | EFFICACY | 2017-09-18 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2017-09-22 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2017-09-22 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2017-09-29 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2017-10-02 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2017-10-02 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2017-10-02 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2017-10-03 |
| AVELUMAB | BAVENCIO | BLA761049 | LABELING | 2017-10-12 |
| IPILIMUMAB | YERVOY | BLA125377 | EFFICACY | 2017-10-20 |
| VEMURAFENIB | ZELBORAF | NDA202429 | EFFICACY | 2017-11-06 |
| BRENTUXIMAB VEDOTIN | ADCETRIS | BLA125388 | EFFICACY | 2017-11-09 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | LABELING | 2017-11-29 |
| POMALIDOMIDE | POMALYST | NDA204026 | LABELING | 2017-11-30 |
| IPILIMUMAB | YERVOY | BLA125377 | LABELING | 2017-12-13 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2017-12-18 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2017-12-18 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2017-12-20 |
| POMALIDOMIDE | POMALYST | NDA204026 | LABELING | 2017-12-29 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-01-09 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-01-09 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-01-09 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-01-09 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-01-09 |
| METHOXSALEN | UVADEX | NDA020969 | LABELING | 2018-01-09 |
| COBIMETINIB FUMARATE | COTELLIC | NDA206192 | EFFICACY | 2018-01-26 |
| PREDNISOLONE SODIUM PHOSPHATE | PEDIAPRED | NDA019157 | LABELING | 2018-02-05 |
| IPILIMUMAB | YERVOY | BLA125377 | LABELING | 2018-02-08 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | LABELING | 2018-02-09 |
| NIVOLUMAB | OPDIVO | BLA125554 | LABELING | 2018-02-13 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-02-15 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-02-15 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-03-05 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-03-05 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-03-05 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-03-05 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-03-05 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-03-05 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-03-05 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-03-05 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-03-05 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-03-05 |
| AMINOLEVULINIC ACID HYDROCHLORIDE | LEVULAN | NDA020965 | EFFICACY | 2018-03-06 |
| TRAMETINIB DIMETHYL SULFOXIDE | MEKINIST | NDA204114 | LABELING | 2018-03-09 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | LABELING | 2018-03-13 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 2018-03-15 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | LABELING | 2018-03-15 |
| BRENTUXIMAB VEDOTIN | ADCETRIS | BLA125388 | EFFICACY | 2018-03-20 |
| POMALIDOMIDE | POMALYST | NDA204026 | LABELING | 2018-03-20 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | LABELING | 2018-03-28 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | LABELING | 2018-03-29 |
| BETAMETHASONE ACETATE | CELESTONE SOLUSPAN | NDA014602 | LABELING | 2018-04-10 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-04-16 |
| IPILIMUMAB | YERVOY | BLA125377 | EFFICACY | 2018-04-16 |
| AMINOLEVULINIC ACID HYDROCHLORIDE | LEVULAN | NDA020965 | LABELING | 2018-04-19 |
| DABRAFENIB MESYLATE | TAFINLAR | NDA202806 | LABELING | 2018-04-20 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 2018-04-23 |
| TRAMETINIB DIMETHYL SULFOXIDE | MEKINIST | NDA204114 | EFFICACY | 2018-04-30 |
| DABRAFENIB MESYLATE | TAFINLAR | NDA202806 | EFFICACY | 2018-04-30 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2018-04-30 |
| INTERFERON ALFA-2B | INTRON A | BLA103132 | LABELING | 2018-05-04 |
| TRAMETINIB DIMETHYL SULFOXIDE | MEKINIST | NDA204114 | EFFICACY | 2018-05-04 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2018-05-04 |
| DABRAFENIB MESYLATE | TAFINLAR | NDA202806 | EFFICACY | 2018-05-04 |
| METHOTREXATE SODIUM | METHOTREXATE PRESERVATIVE FREE | NDA011719 | LABELING | 2018-05-07 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2018-05-25 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2018-06-12 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2018-06-13 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2018-06-14 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2018-06-19 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2018-06-19 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | LABELING | 2018-06-22 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | LABELING | 2018-06-26 |
| ENCORAFENIB | BRAFTOVI | NDA210496 | TYPE 1/4 | 2018-06-27 |
| BINIMETINIB | MEKTOVI | NDA210498 | TYPE 1/4 | 2018-06-27 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2018-07-02 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-07-10 |
| IPILIMUMAB | YERVOY | BLA125377 | EFFICACY | 2018-07-10 |
| METHYLPREDNISOLONE ACETATE | DEPO-MEDROL | NDA011757 | LABELING | 2018-07-24 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | LABELING | 2018-07-24 |
| METHYLPREDNISOLONE SODIUM SUCCINATE | SOLU-MEDROL | NDA011856 | LABELING | 2018-07-24 |
| MOGAMULIZUMAB-KPKC | POTELIGEO | BLA761051 | TYPE 1 | 2018-08-08 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2018-08-16 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2018-08-16 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2018-08-20 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2018-08-21 |
| CEMIPLIMAB-RWLC | LIBTAYO | BLA761097 | TYPE 1 | 2018-09-28 |
| AVELUMAB | BAVENCIO | BLA761049 | EFFICACY | 2018-10-19 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2018-10-30 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2018-11-09 |
| NIVOLUMAB | OPDIVO | BLA125554 | LABELING | 2018-11-15 |
| BRENTUXIMAB VEDOTIN | ADCETRIS | BLA125388 | EFFICACY | 2018-11-16 |
| ROMIDEPSIN | ISTODAX | NDA022393 | LABELING | 2018-11-27 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2018-12-06 |
| VORINOSTAT | ZOLINZA | NDA021991 | LABELING | 2018-12-11 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2018-12-19 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2018-12-21 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2018-12-28 |
| PEGINTERFERON ALFA-2B | PEGINTRON | BLA103949 | LABELING | 2019-01-08 |
| VISMODEGIB | ERIVEDGE | NDA203388 | EFFICACY | 2019-01-18 |
| CEMIPLIMAB-RWLC | LIBTAYO | BLA761097 | LABELING | 2019-01-18 |
| ENCORAFENIB | BRAFTOVI | NDA210496 | EFFICACY | 2019-01-23 |
| BINIMETINIB | MEKTOVI | NDA210498 | EFFICACY | 2019-01-23 |
| NIVOLUMAB | OPDIVO | BLA125554 | LABELING | 2019-02-01 |
| DENILEUKIN DIFTITOX | ONTAK | BLA103767 | LABELING | 2019-02-15 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2019-02-15 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2019-03-07 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2019-03-07 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2019-03-08 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2019-03-18 |
| CEMIPLIMAB-RWLC | LIBTAYO | BLA761097 | LABELING | 2019-03-20 |
| VISMODEGIB | ERIVEDGE | NDA203388 | LABELING | 2019-03-26 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2019-04-11 |
| TRIAMCINOLONE ACETONIDE | KENALOG-40 | NDA014901 | MANUF (CMC) | 2019-04-12 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2019-04-18 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2019-04-19 |
| NIVOLUMAB | OPDIVO | BLA125554 | LABELING | 2019-05-02 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2019-05-06 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2019-05-06 |
| DEXAMETHASONE | DECADRON | NDA011664 | LABELING | 2019-05-08 |
| IPILIMUMAB | YERVOY | BLA125377 | LABELING | 2019-05-08 |
| SONIDEGIB PHOSPHATE | ODOMZO | NDA205266 | LABELING | 2019-05-13 |
| AVELUMAB | BAVENCIO | BLA761049 | EFFICACY | 2019-05-14 |
| ALDESLEUKIN | PROLEUKIN | BLA103293 | LABELING | 2019-05-17 |
| ENCORAFENIB | BRAFTOVI | NDA210496 | EFFICACY | 2019-05-24 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2019-06-10 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2019-06-17 |
| TRAMETINIB DIMETHYL SULFOXIDE | MEKINIST | NDA204114 | LABELING | 2019-07-16 |
| DABRAFENIB MESYLATE | TAFINLAR | NDA202806 | LABELING | 2019-07-16 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2019-07-22 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2019-07-30 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2019-07-30 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 2019-08-09 |
| DOXORUBICIN HYDROCHLORIDE | DOXIL (LIPOSOMAL) | NDA050718 | LABELING | 2019-08-12 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | MANUF (CMC) | 2019-08-16 |
| METHYLPREDNISOLONE | MEDROL | NDA011153 | MANUF (CMC) | 2019-08-26 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2019-09-17 |
| NIVOLUMAB | OPDIVO | BLA125554 | LABELING | 2019-09-18 |
| IPILIMUMAB | YERVOY | BLA125377 | LABELING | 2019-09-20 |
| TRAMETINIB DIMETHYL SULFOXIDE | MEKINIST | NDA204114 | EFFICACY | 2019-10-06 |
| DABRAFENIB MESYLATE | TAFINLAR | NDA202806 | EFFICACY | 2019-10-06 |
| BRENTUXIMAB VEDOTIN | ADCETRIS | BLA125388 | LABELING | 2019-10-15 |
| POMALIDOMIDE | POMALYST | NDA204026 | LABELING | 2019-10-30 |
| HYDROCORTISONE | CORTEF | NDA008697 | LABELING | 2019-11-21 |
| HYDROCORTISONE SODIUM SUCCINATE | SOLU-CORTEF | NDA009866 | LABELING | 2019-11-21 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2019-12-03 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2019-12-18 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 2019-12-20 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-01-08 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | LABELING | 2020-01-09 |
| MECHLORETHAMINE HYDROCHLORIDE | VALCHLOR | NDA202317 | LABELING | 2020-01-13 |
| DENILEUKIN DIFTITOX | ONTAK | BLA103767 | LABELING | 2020-02-10 |
| INGENOL MEBUTATE | PICATO | NULL | LABELING | 2020-02-13 |
| PREDNISOLONE SODIUM PHOSPHATE | ORAPRED ODT | NDA021959 | LABELING | 2020-03-06 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2020-03-10 |
| IPILIMUMAB | YERVOY | BLA125377 | EFFICACY | 2020-03-10 |
| ENCORAFENIB | BRAFTOVI | NDA210496 | EFFICACY | 2020-04-08 |
| TRAMETINIB DIMETHYL SULFOXIDE | MEKINIST | NDA204114 | LABELING | 2020-04-09 |
| DABRAFENIB MESYLATE | TAFINLAR | NDA202806 | LABELING | 2020-04-09 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-04-28 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-04-28 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-04-28 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-04-28 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-04-28 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-04-28 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-04-28 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-04-28 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-04-28 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-04-28 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-04-28 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-04-28 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-04-28 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-04-28 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-04-28 |
| MECHLORETHAMINE HYDROCHLORIDE | VALCHLOR | NDA202317 | LABELING | 2020-05-01 |
| METHOTREXATE SODIUM | METHOTREXATE SODIUM | NDA008085 | EFFICACY | 2020-05-04 |
| POMALIDOMIDE | POMALYST | NDA204026 | EFFICACY | 2020-05-14 |
| POMALIDOMIDE | POMALYST | NDA204026 | EFFICACY | 2020-05-14 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2020-05-15 |
| IPILIMUMAB | YERVOY | BLA125377 | EFFICACY | 2020-05-15 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2020-05-18 |
| VEMURAFENIB | ZELBORAF | NDA202429 | LABELING | 2020-05-18 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2020-05-26 |
| IPILIMUMAB | YERVOY | BLA125377 | EFFICACY | 2020-05-26 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2020-05-29 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2020-06-10 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-06-16 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-06-16 |
| TRAMETINIB DIMETHYL SULFOXIDE | MEKINIST | NDA204114 | LABELING | 2020-06-23 |
| NIVOLUMAB | OPDIVO | BLA125554 | LABELING | 2020-06-23 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-06-24 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-06-24 |
| CEMIPLIMAB-RWLC | LIBTAYO | BLA761097 | EFFICACY | 2020-06-25 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-06-29 |
| IPILIMUMAB | YERVOY | BLA125377 | LABELING | 2020-06-29 |
| AVELUMAB | BAVENCIO | BLA761049 | EFFICACY | 2020-06-30 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2020-07-30 |
| VISMODEGIB | ERIVEDGE | NDA203388 | LABELING | 2020-07-31 |
| CYCLOPHOSPHAMIDE | CYTOXAN | NDA012141 | MANUF (CMC) | 2020-08-07 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2020-08-10 |
| IMATINIB MESYLATE | GLEEVEC | NDA021588 | LABELING | 2020-08-10 |
| IPILIMUMAB | YERVOY | BLA125377 | LABELING | 2020-08-13 |
| DOXORUBICIN HYDROCHLORIDE | DOXORUBICIN HYDROCHLORIDE | NDA050467 | LABELING | 2020-08-28 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2020-09-18 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2020-09-18 |
| IPILIMUMAB | YERVOY | BLA125377 | EFFICACY | 2020-09-18 |
| NIVOLUMAB | OPDIVO | BLA125554 | LABELING | 2020-09-25 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2020-09-30 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2020-10-02 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-10-02 |
| IPILIMUMAB | YERVOY | BLA125377 | EFFICACY | 2020-10-02 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-10-14 |
| AVELUMAB | BAVENCIO | BLA761049 | LABELING | 2020-11-10 |
| CEMIPLIMAB-RWLC | LIBTAYO | BLA761097 | LABELING | 2020-11-10 |
| NIVOLUMAB | OPDIVO | BLA125554 | LABELING | 2020-11-10 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | LABELING | 2020-11-10 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | LABELING | 2020-11-10 |
| PEMBROLIZUMAB | KEYTRUDA | BLA125514 | EFFICACY | 2020-11-13 |
| IPILIMUMAB | YERVOY | BLA125377 | LABELING | 2020-11-13 |
| POMALIDOMIDE | POMALYST | NDA204026 | EFFICACY | 2020-11-20 |
| POMALIDOMIDE | POMALYST | NDA204026 | LABELING | 2020-12-03 |
| TIRBANIBULIN | KLISYRI | NDA213189 | TYPE 1 | 2020-12-14 |
| INGENOL MEBUTATE | PICATO | NULL | LABELING | 2020-12-17 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2020-12-18 |
| ATEZOLIZUMAB | TECENTRIQ | BLA761034 | EFFICACY | 2020-12-18 |
| NIVOLUMAB | OPDIVO | BLA125554 | LABELING | 2020-12-29 |
| NIVOLUMAB | OPDIVO | BLA125554 | EFFICACY | 2021-01-22 |
| HYDROXYUREA | DROXIA | NDA016295 | LABELING | 2021-02-09 |
| CEMIPLIMAB-RWLC | LIBTAYO | BLA761097 | EFFICACY | 2021-02-09 |
| CEMIPLIMAB-RWLC | LIBTAYO | BLA761097 | EFFICACY | 2021-02-09 |
Table 2. Label Modifications to Therapeutics with an Indication in Skin Cancer
The above table lists all labeling modifications for the therapeutic agents (or agents) with an indication for skin cancer. Abbreviations - TYPE1: Type 1 - New Molecular Entity; TYPE2: Type 2 - New Active Ingredient; TYPE3 - New Dosage Form; TYPE4: Type 4 - New Combination; TYPE5: Type 5 - New Formulation or New Manufacturer; MANUF (CMC): Chemistry, Manufacturing and Controls.